User:Mr. Ibrahem/Trimipramine
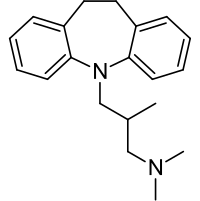 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Surmontil, others |
| Other names | Trimeproprimine; IF-6120; IL-6001; RP-7162; 2'-Methylimipramine; β-Methylimipramine |
| AHFS/Drugs.com | |
| MedlinePlus | a602010 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular injection, intravenous |
| Drug class | Tricyclic antidepressant (TCA)[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 41%[2][3][4][5] |
| Protein binding | 94.9%[2][3][4][5] |
| Metabolism | Liver[2][3][4][5] |
| Elimination half-life | 23–24 hours[2][3][4][5] |
| Excretion | Kidney[2][3][4][5] |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C20H26N2 |
| Molar mass | 294.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Trimipramine, sold under the brand name Surmontil among others, is a medication primarily used to treat major depressive disorder.[1] Other uses may include trouble sleeping and bedwetting in children.[1] It is taken by mouth.[1] Benefits may required more than two weeks to occur.[1]
Common side effects include sleepiness, dry mouth, constipation, low blood pressure with standing, and increased weight.[1] Other side effects may include high blood sugar, liver problems, suicide, and sexual dysfunction.[6] It is a tricyclic antidepressant (TCA); however, how it works is not entirely clear.[1]
was approved for medical use in the United States in 1979.[1] It is available as a generic medication.[6] In the United Kingdom 28 tablets of 50 mg costs the NHS about 220 pounds as of 2021.[6] This amount in the United States costs about 50 USD.[7]
References[edit]
- ^ a b c d e f g h i j "Trimipramine Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 20 September 2021.
- ^ a b c d e "PRODUCT INFORMATION SURMONTIL® Tablets and Capsules" (PDF). TGA eBusiness Services. Aspen Pharmacare Australia Pty Ltd. 28 November 2012. Archived from the original on 1 November 2016. Retrieved 30 November 2013.
- ^ a b c d e "SURMONTIL (trimipramine maleate) capsule [Duramed Pharmaceuticals Inc]". DailyMed. Duramed Pharmaceuticals Inc. December 2012. Archived from the original on 4 July 2013. Retrieved 30 November 2013.
- ^ a b c d e "Surmontil, Trimip (trimipramine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 3 December 2013. Retrieved 30 November 2013.
- ^ a b c d e "Trimipramine 50mg Capsules - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Zentiva. 19 November 2012. Archived from the original on 3 December 2013. Retrieved 30 November 2013.
- ^ a b c d BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 396. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Trimipramine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 14 June 2016. Retrieved 20 September 2021.
