User:Mr. Ibrahem/Tolazamide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tolinase, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682482 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Sulfonylurea[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | metabolized in the liver to active metabolites |
| Onset of action | Within 20 min[1] |
| Elimination half-life | 7 hours |
| Duration of action | 10 hrs[1] |
| Excretion | Kidney (85%) and fecal (7%) |
| Identifiers | |
| |
| Chemical and physical data | |
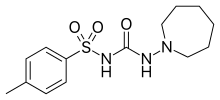
| Formula | C14H21N3O3S |
| Molar mass | 311.40 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tolazamide, sold under the brand name Tolinase among others, is a medication used to treat type 2 diabetes.[1] It is taken by mouth.[1] Effects begin within 20 minutes and last for about 10 hours.[1]
Common side effects include nausea, loss of appetite, diarrhea, and abdominal pain.[1] Other side effects may include rash and low blood sugar.[1] Side effects are more common in people with liver or kidney problems.[1] It is a sulfonylurea.[1]
Tolazamide was approved for medical use in the United States in 1966.[1] It is available as a generic medication.[2] In the United States 90 tablets of 250 mg costs about 54 USD.[3]
References[edit]
- ^ a b c d e f g h i j k l m "Tolazamide Monograph for Professionals". Drugs.com. Archived from the original on 4 September 2019. Retrieved 5 October 2021.
- ^ Skyler, Jay (4 April 2012). Atlas of Diabetes. Springer Science & Business Media. p. 186. ISBN 978-1-4614-1027-0. Archived from the original on 9 October 2021. Retrieved 5 October 2021.
- ^ "Tolazamide Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 5 October 2021.
