User:Mr. Ibrahem/Sodium oxybate
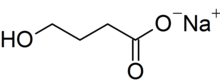 | |
| Clinical data | |
|---|---|
| Trade names | Xyrem, Alcover, Somsanit, others[1] |
| Other names | γ-Hydroxybutyric acid (GHB), 4-hydroxybutanoic acid, NSC-84223, WY-3478 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605032 |
| License data | |
| Addiction liability | Extremely high[2] |
| Routes of administration | By mouth, intravenous |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 88%[3] |
| Protein binding | <1%[3] |
| Onset of action | Within 30 min[4] |
| Elimination half-life | 0.5 to 1 hour. |
| Excretion | Almost entirely by biotransformation to carbon dioxide, which is then eliminated by expiration |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C4H7NaO3 |
| Molar mass | 126.087 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Sodium oxybate, also known as γ-Hydroxybutyric acid (GHB), is a medication used to treat sudden muscle weakness and excessive daytime sleepiness seen in narcolepsy.[3] It may also be used for anesthesia, alcohol withdrawal, and opioid withdrawal.[5][4] It is taken by mouth or by injection into a vein.[9][5] Effects begin within 30 minutes.[4]
Common side effects include nausea, dizziness, sleepiness, and tremor.[6] Other side effects may include respiratory depression (insufficient breathing), misuse, seizures, and coma.[3] It should not be used with alcohol.[3] Use is not recommended in pregnancy or breastfeeding.[10] It works via GABA receptors.[4]
Sodium oxybate was approved for medical use in the United States in 2002 and Europe in 2005.[6][8] Though it has been used since the 1960s for a number of conditions.[5] It is available as a generic medication.[9] In the United Kingdom 90 mg costs the NHS about £360 as of 2021.[9] This amount in the United States costs about 5,800 USD.[11] It is sold under the brand name Xyrem among others.[3] In Canada and United States it is classified as Schedule III, while in Europe it is a Schedule IV controlled substance.[12] It has also been used recreationally at raves.[4]
References[edit]
- ^ "International brands for Sodium Oxybate -". Drugs.com. Archived from the original on 16 April 2018. Retrieved 16 April 2018.
- ^ Fisher, Gary L.; Roget, Nancy A. (2009). Encyclopedia of Substance Abuse Prevention, Treatment, and Recovery. SAGE. p. PT136. ISBN 978-1-4129-5084-8. Archived from the original on 2021-10-17. Retrieved 2021-10-12.
- ^ a b c d e f g h "Xyrem- sodium oxybate solution". DailyMed. 30 September 2020. Archived from the original on 12 November 2020. Retrieved 14 November 2020.
- ^ a b c d e M, Procyshyn, Ric; Z, Bezchlibnyk-Butler, Kalyna; Joel, Jeffries, J. (15 July 2021). Clinical Handbook of Psychotropic Drugs. Hogrefe Publishing. p. 378. ISBN 978-1-61676-593-4. Archived from the original on 17 October 2021. Retrieved 12 October 2021.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d "Critical review of gamma-hydroxybutyric acid (GHB)" (PDF). 2012. Archived (PDF) from the original on 2021-03-12. Retrieved 2021-06-27.: 15, 27–28
- ^ a b c "Sodium Oxybate Monograph for Professionals". Drugs.com. Archived from the original on 14 November 2020. Retrieved 12 October 2021.
- ^ "Sodium oxybate (Xyrem) Use During Pregnancy". Drugs.com. 2 April 2020. Archived from the original on 22 July 2020. Retrieved 22 July 2020.
- ^ a b "Xyrem". Archived from the original on 6 October 2021. Retrieved 12 October 2021.
- ^ a b c BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 513. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Sodium oxybate (Xyrem) Use During Pregnancy". Drugs.com. Archived from the original on 22 July 2020. Retrieved 12 October 2021.
- ^ "Xyrem Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 15 January 2021. Retrieved 12 October 2021.
- ^ Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL (August 2009). "Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion". Journal of Clinical Sleep Medicine. 5 (4): 365–71. doi:10.5664/jcsm.27549. PMC 2725257. PMID 19968016.
