User:Mr. Ibrahem/Olanzapine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zyprexa, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601213 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular injection |
| Drug class | Atypical antipsychotic[2] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60-65%[3][4][5] |
| Protein binding | 93%[6] |
| Metabolism | Liver (direct glucuronidation and CYP1A2 mediated oxidation) |
| Elimination half-life | 33 hours, 51.8 hours (elderly)[6] |
| Excretion | Urine (57%; 7% as unchanged drug), faeces (30%)[6][7] |
| Identifiers | |
| |
| Chemical and physical data | |
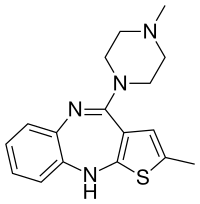
| Formula | C17H20N4S |
| Molar mass | 312.439 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 195 °C (383 °F) |
| Solubility in water | Practically insoluble in water mg/mL (20 °C) |
| |
| |
| | |
Olanzapine, sold under the trade name Zyprexa among others, is an atypical antipsychotic primarily used to treat schizophrenia and bipolar disorder.[2] For schizophrenia, it can be used for both new onset disease and long-term maintenance.[2] It is taken by mouth or by injection into a muscle.[2]
Common side effects include weight gain, movement disorders, dizziness, feeling tired, constipation, and dry mouth.[2] Other side effects include low blood pressure with standing, allergic reactions, neuroleptic malignant syndrome, high blood sugar, seizures, gynecomastia, erectile dysfunction, and tardive dyskinesia.[2] In older people with dementia, its use increases the risk of death.[2] Use in the later part of pregnancy may result in a movement disorder in the baby for some time after birth.[2] Although how it works is not entirely clear, it blocks dopamine and serotonin receptors.[2]
Olanzapine was patented in 1971 and approved for medical use in the United States in 1996.[2][10] It is available as a generic medication.[2] It is on the World Health Organization's List of Essential Medicines.[11] In the United States, the wholesale cost is less than US$0.25 per dose as of 2018.[12] In 2017, it was the 239th most commonly prescribed medication in the United States, with more than two million prescriptions.[13][14]
References[edit]
- ^ Drugs.com Drugs.com international listings for Olanzapine Archived 2017-07-11 at the Wayback Machine Page accessed August 4, 2015
- ^ a b c d e f g h i j k l "Olanzapine, Olanzapine Pamoate Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 6 November 2018. Retrieved 24 December 2018.
- ^ Kassahun, Kelem; Mattiuz, Edward; Nyhart, Eldon (January 1, 1997). "DISPOSITION AND BIOTRANSFORMATION OF THE ANTIPSYCHOTIC AGENT OLANZAPINE IN HUMANS". Drug Metabolism and Disposition. 25 (1): 81–93. PMID 9010634. Archived from the original on February 22, 2019. Retrieved February 22, 2019.
- ^ Callaghan JT, Bergstrom RF, Ptak LR, et al. (September 1999). "Olanzapine: pharmacokinetic and pharmacodynamic profile". Clin Pharmacokinetics. 37 (3): 177–193. doi:10.2165/00003088-199937030-00001. PMID 10511917.
- ^ Mauri MC, Volonteri LS, Colasanti A, et al. (2007). "Clinical pharmacokinetics of atypical antipsychotics: a critical review of the relationship between plasma concentrations and clinical response". Clinical Pharmacokinetics. 46 (5): 359–88. doi:10.2165/00003088-200746050-00001. PMID 17465637. S2CID 43859718.
- ^ a b c "PRODUCT INFORMATION OLANZAPINE SANDOZ® 2.5mg/5mg/7.5mg/10mg/15mg/20mg FILM-COATED TABLETS" (PDF). TGA eBusiness Services. Sandoz Pty Ltd. 8 June 2012. Archived from the original on 17 October 2015. Retrieved 26 November 2013.
- ^ "Zyprexa, Zyprexa Relprevv (olanzapine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 2 December 2013. Retrieved 26 November 2013.
- ^ "OLANZAPINE oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 28 August 2021. Retrieved 1 September 2020.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 6 November 2020. Retrieved 1 September 2020.
- ^ Taylor D, Paton C, Kapur S (2015). The Maudsley Prescribing Guidelines in Psychiatry (12th ed.). London, U K: Wiley-Blackwell. p. 16. ISBN 978-1-118-75460-3.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "NADAC as of 2018-12-19". Centers for Medicare and Medicaid Services. Archived from the original on 2018-12-19. Retrieved 22 December 2018.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Olanzapine - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
