User:Mr. Ibrahem/Nimodipine
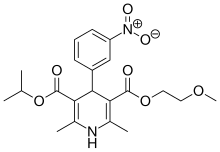 | |
| Clinical data | |
|---|---|
| Trade names | Nimotop, Nymalize, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a689010 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, by mouth |
| Drug class | Calcium channel blocker (dihydropyridine) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 13% (by mouth) |
| Protein binding | 95% |
| Metabolism | Liver |
| Onset of action | Rapid[2] |
| Elimination half-life | 8–9 hours |
| Duration of action | 4 hrs[2] |
| Excretion | Feces and Urine |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C21H26N2O7 |
| Molar mass | 418.446 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 7 °C (45 °F) |
| |
| |
| (verify) | |
Nimodipine, sold under the brand name Nimotop among others, is a medication used to prevent vasospasm secondary to subarachnoid hemorrhage.[3] It is taken by mouth or by injection into a vein.[3][4] Onset is rapid with a duration of action of 4 hours.[2]
Common side effects include low blood pressure and headache.[3] Other side effects may include slow heart rate, ileus, and low platelets.[4] Safety in pregnancy is unclear.[5] It is a calcium channel blocker of the dihydropyridine type.[3]
Nimodipine was patented in 1971 and approved for medical use in Germany in 1985.[6][7] It was approved in the United States in 1988.[3] In the United Kingdom 100 tablets of 30 mg costs the NHS about £40.[4] This amount in the United States costs about 170 USD.[8]
References[edit]
- ^ a b "Nimodipine Use During Pregnancy". Drugs.com. March 15, 2019. Archived from the original on September 21, 2021. Retrieved April 11, 2020.
- ^ a b c Frishman, William H.; Cheng-Lai, Angela; Chen, Julie (June 29, 2013). Current Cardiovascular Drugs. Springer Science & Business Media. p. 161. ISBN 978-1-4615-6767-7. Archived from the original on November 13, 2021. Retrieved November 13, 2021.
- ^ a b c d e f g h "Nimodipine Monograph for Professionals". Drugs.com. Archived from the original on September 21, 2021. Retrieved November 13, 2021.
- ^ a b c BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 124. ISBN 978-0857114105.
- ^ "Nimodipine Use During Pregnancy". Drugs.com. Archived from the original on September 21, 2021. Retrieved November 13, 2021.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 464. ISBN 9783527607495. Archived from the original on August 29, 2021. Retrieved October 15, 2021.
- ^ Bayer AG of Germany (April 10, 1971). "New molecular entity with antihypertensive properties" (Patent (Post-Approval)). UK Patent Office / EspaceNet Patent Search. British patent 1,358,951: Patent Office of the United Kingdom. p. GB1358951. Archived from the original on November 12, 2020. Retrieved April 11, 2019.
Priority date: 1971-04-10 (...) Date issued: 1974-07-03
{{cite web}}: CS1 maint: location (link) - ^ "Nimodipine Prices and Nimodipine Coupons - GoodRx". GoodRx. Retrieved November 13, 2021.
