User:Mr. Ibrahem/Eslicarbazepine acetate
 | |
| Clinical data | |
|---|---|
| Trade names | Aptiom, Zebinix, Exalief, others |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| Drug class | Anticonvulsant |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | ~30%[4] |
| Metabolism | UGT (?) |
| Metabolites | Eslicarbazepine (active), glucuronides (inactive), etc. |
| Elimination half-life | 10–20 hours |
| Excretion | ~90% renal |
| Identifiers | |
| |
| Chemical and physical data | |
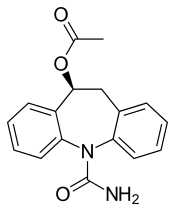
| Formula | C17H16N2O3 |
| Molar mass | 296.326 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Eslicarbazepine acetate (ESL), sold under the brand names Aptiom and Zebinix among others, is a medication used to treat epilepsy with focal-onset seizures.[3][2] It is taken by mouth.[2] It may be used along or with other anti seizure medications.[5]
Common side effects include dizziness, sleepiness, nausea, headache, double vision, tiredness, poor coordination, blurry vision, and tremor.[2] Other side effects may include suicide, anaphylaxis, low sodium, and liver problems.[2] Safety in pregnancy is unclear.[6] It is a prodrug to (S)-(+)-licarbazepine, similarly to oxcarbazepine; and is beleived to work by inhibiting sodium channels.[6]
Eslicarbazepine acetate was approved for medical use in Europe in 2009 and the United States in 2013.[6][3] In the United Kingdom a dose of 400 mg per day for a month costs the NHS about £68 as of 2021.[5] In the United States this amount costs about 1,050 USD.[7]
References[edit]
- ^ a b "Zebinix". Therapeutic Goods Administration (TGA). 9 June 2021. Archived from the original on 6 September 2021. Retrieved 6 September 2021.
- ^ a b c d e f "Aptiom- eslicarbazepine acetate tablet Aptiom- eslicarbazepine acetate kit". DailyMed. Archived from the original on 27 March 2021. Retrieved 21 January 2021.
- ^ a b c d "Zebinix EPAR". European Medicines Agency (EMA). Archived from the original on 5 March 2021. Retrieved 21 January 2021.
- ^ Dinnendahl V, Fricke U, eds. (2011). Arzneistoff-Profile (in German). Vol. 4 (25 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ^ a b BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 330. ISBN 978-0857114105.
- ^ a b c "Eslicarbazepine Monograph for Professionals". Drugs.com. Archived from the original on 18 January 2021. Retrieved 16 December 2021.
- ^ "Eslicarbazepine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 16 December 2021.
