User:Mr. Ibrahem/Dantrolene
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Dantrium, Revonto, Ryanodex |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682576 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 70% |
| Metabolism | Liver |
| Excretion | Biliary, kidney |
| Identifiers | |
| |
| Chemical and physical data | |
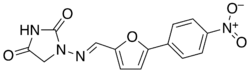
| Formula | C14H10N4O5 |
| Molar mass | 314.257 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dantrolene sodium, sold under the brand name Dantrium among others, is a medication used to treat malignant hyperthermia, neuroleptic malignant syndrome, and muscle spasticity.[3][4] This may include spasticity such as due to multiple sclerosis, cerebral palsy, or stroke.[4] Other possible uses include MDMA toxicity.[5] It may be used by injection into a vein or by mouth.[3]
Common side effects include sleepiness, dizziness, weakness, and diarrhea.[4] Intravenous use may also result in inflammation at the site.[4] Other side effects may include liver problems or palpitations.[3] It works by blocking Ca2+ ions release by skeletal muscles and thereby preventing their contraction.[3]
Dantrolene was first made in 1967 by Snyder.[5] It was approved for medical use in the United States in 1974.[4] It is available as a generic medication.[6] Hospitals are recommended to keep at least 36 vials totaling 720 mg on hand, enough for a 70-kg person.[7] This amount in the United Kingdom costs the NHS about £1,800 and in the United States about 3,000 USD as of 2020.[3][8]
References[edit]
- ^ a b "Dantrolene Use During Pregnancy". Drugs.com. 9 December 2019. Archived from the original on 25 January 2021. Retrieved 6 July 2020.
- ^ "Dantrium 25mg Capsules - Summary of Product Characteristics (SmPC)". (emc). 28 February 2020. Archived from the original on 7 July 2020. Retrieved 6 July 2020.
- ^ a b c d e f BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1417. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ a b c d e f g "Dantrolene Monograph for Professionals". Drugs.com. Archived from the original on 28 January 2021. Retrieved 21 July 2021.
- ^ a b Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F (2004). "Dantrolene – a review of its pharmacology, therapeutic use and new developments". Anaesthesia. 59 (4): 364–73. doi:10.1111/j.1365-2044.2004.03658.x. PMID 15023108. S2CID 18537509.
- ^ "Dantrolene Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 13 October 2016. Retrieved 21 July 2021.
- ^ Yeung EY, Munroe J (2015). "Development of a malignant hyperthermia protocol". BMC Proceedings. 9 (Suppl1): A32. doi:10.1186/1753-6561-9-S1-A32. PMC 4306034.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ho, PT; Carvalho, B; Sun, EC; Macario, A; Riley, ET (August 2018). "Cost-benefit Analysis of Maintaining a Fully Stocked Malignant Hyperthermia Cart versus an Initial Dantrolene Treatment Dose for Maternity Units". Anesthesiology. 129 (2): 249–259. doi:10.1097/ALN.0000000000002231. PMID 29672336.
