User:Ccoyiuto/sandbox
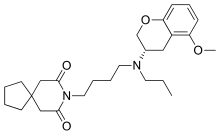 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H38N2O4 |
| Molar mass | 442.589 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Alnespirone (S-20,499) is a selective 5-HT1A receptor full agonist of the azapirone chemical class.[1][2][3] It has antidepressant, anti-aggression and anxiolytic effects in animal models [1]. Phase II trials have been performed in Europe, but development was discontinued [4] [5].
History[edit]

Discovery of Alnespirone’s (S-20499) selective agonistic binding to 5-HT1A began with studies of chroman derivative 5-methoxy-3-(di-n-propylamino)chroman, which was found to act as an agonist on 5-HT1A receptors [6]. This subsequently led to synthesis of other chroman derivatives such as S-20244, which has both high affinity and selectivity for 5-HT1A receptors [6]. Amongst racemic (+/-)-S-20244 and its enantiomers (+)-S-20499 and (-)-S-20500, (+)-S-20499 yields the highest affinity for 5-HT1A [6].
Pharmacokinetics[edit]
Alnespirone is an aminochroman derivative of the azapirone family [6]. It contains a piperazine nucleus, which is commonly found in other antidepressant and anxiolytic drugs such as gepipirone, ipsapirone and buspirone which also act on 5HT1A receptors [7].
Alnespirone shares structural similarities to other 5-HT receptor agonists 8-OH-DPAT and buspirone [8] [3] [9]. Because it does not metabolize into 1-(2-pyrimidinyl)-piperazine, which antagonizes α2-adrenoceptors, Alnespirone does not exert any affect on catecholaminergic neurons [10] [3]. The drug is lipophilic with a partition coefficient of log P > 2, so can easily pass through the blood brain barrier [9].
Anxiolytic activity is produced at lower doses (<1 mg/kg/day in Wistar rats) and antidepressant activity at midrange doses (1-5 mg/kg/day in Wistar rats) [11]. Alnespirone also has an ED50 of 0.3 mg kg-1 in the DRN, MRN, STR, FC, DHPC and VHPC [10]. It has a longer half-life than fellow 5-HT1A agonist 8-OH-DPAT [10].
From animal model studies, Alnespirone is usually administered intraperitoneally or subcutaneously in rats, and intramuscularly in monkeys [11] [10] [8] [12].
In a clinical trial, Alnespirone was administered orally [13].
Pharmacodynamics[edit]
In vitro, the drug acts as a selective full presynaptic and postsynaptic agonist, and in vivo, full presynaptic and partial postsynaptic agonist [11]. If acting on the 5-HT1A autoreceptors, Alnespirone has anti-aggressive effects as the drug inhibits firing of 5-HT neurons and 5-HT release [14]. Conversely, when acting on postsynaptic receptors, Alnespirone promotes 5-HT release at doses that otherwise reduce aggression when acting presynaptically [15] [14].
The drug displays low affinity for other 5-HT receptor binding sites such as 5-HT1B (IC50 = 5-10 uM) and 5-HT2 (IC50 = 1-5 uM) [6].
Rat studies demonstrate that Alnespirone is regionally selective, reducing extracellular 5-HT in the striatum (maximal reduction to 23% of baseline), frontal cortex (29%), dorsal and ventral hippocampus (65%), median raphe nucleus (30%) and dorsal raphe nucleus (60%) [10] [8].
Desensitization and Down Regulation of 5-HT1A[edit]
Alnespirone is capable of desensitizing the somatodendritic 5-HT1A receptors in the dorsal raphe nucleus and frontal cortex [8] [16]. In rats, Alnespirone is capable of decreasing the potency of 8-OH-DPAT inhibition in DRN HT1A autoreceptors, but this Alnespirone-mediated desensitization takes longer to develop that that mediated by agonists ipsapirone and depirone [16].
Autoradiographic experiments in rats also show a reduction in the somatodendritic 5-HT1A receptors, but no such down regulation is observed in the postsynaptic receptors when treated with Alnespirone[8].
Potency[edit]
Alnespirone has a Ki = 0.19 nM at 5-HT1A hippocampal receptors [6], and its radioligand [3H] Alnespirone has a Kd = 0.36 nM in rat hippocampal membrane [17]. The drug has an anti-aggressive ID50 of 1.24 mg/kg [18].
Efficacy[edit]
The drug has an inverted U-shaped dose dependent relationship in the Chronic Mild Stress Model of Depression as demonstrated in rats, in which there was a lack of significant effect for low doses (0.5 mg/kg/day) and high doses (10, 20 mg/kg/day) [11].
Treatment[edit]
Studies primarily using animal models have demonstrated how Alnespirone could serve as a potential drug to treat symptoms of depression, aggression, and anxiety.
Depression[edit]
5-HT1A agonists such as Alnespirone are able to produce anti-depressive effects, but there is still debate on whether the drug acts on presynaptic or postsynaptic receptors [11].
Other studies demonstrate Alnespirone’s anti-depressive properties through the drug’s indirect regulation of certain receptors. β1-adrenoceptors are reportedly more abundant in depressed patients, and upon antidepressant treatment usually decrease in number [19]. Similarly, 5-HT2 receptors have been found to increase in density in the cortex of depressed suicide victims [19]. Anti-depressive activity was demonstrated in chronic treatment of Alnespirone in rats, which produced a slight decrease in β1-adrenoceptor density, and significantly reduced 5-HT2 density in the cortex [12].
Anti-Aggression[edit]
Drugs that target the 5-HT1A receptors have been found to alter aggressive behavior [18]. Studies using a resident-intruder agonistic paradigm in Alnespirone treated rats showed a dose-dependent reduction in aggressive actions (less threat, attacks and pursuit towards an intruder) [15] [18].
The 5-HT syndrome is often induced in rats by drugs that act as agonists on 5-HT1A receptors [9]. Anti-aggressive studies on Alnespirone show that the drug produces hypothermia, but unlike other 5-HT1A agonists 8-OH-DPAT, buspirone, ipsapirone and 5-MeODMT, Alnespirone does not yield the other sedative side effects characterized by the syndrome [15] [9]. Instead, the reduction in anti-aggressive behavior induced by Alnespirone also produced significant increases in social exploration [14]. Research suggests that this may be because the drug does not affect postsynaptic 5-HT1A receptors at anti-aggressive doses [14], or may selectively act on subtypes of the 5-HT1A receptors [9]. Action at subtypes of 5-HT1A was proposed as Alnespirone does not decrease or induce forepaw treading in rats unlike other 5-HT1A agonists [9]. Because the dose of Alnespirone (75-120 mg/kg) in forepaw treading studies is much larger than the EC50 hypothermia-inducing dose, Alnespirone may preferentially bind to the 5-HT1A subtype mediating hypothermia [9].
Anxiety[edit]
Agonistic effects mediated by Alnespirone on presynaptic 5-HT1A receptors appear to produce anxiolytic properties [19]. Mice treated with 4-63 mg/kg i.p of Alnespirone experienced symptoms of sedation such as hypoactivity and decrease in traction and muscle tone [20]. At 4 mg/kg, there was an increase in number of shocks taken in the Vogel conflict test and at 16 mg/kg, greater immobility was found in the tail suspension test, indicative of increased anxiolytic activity [20].
In a clinical trial, Alnespirone was able to induce dose-dependent sedation in healthy volunteers as demonstrated through EEG mapping and psychometric measures [13].
Side Effects[edit]
Alnespirone has been found to increase melatonin production at night, suggesting how its effects on 5-HT1A receptors may influence the sleep/wake cycle [21].
In addition, studies using animal models demonstrate how Alnespirone increases levels of oxytocin, ACTH and dopamine in rats [17] [22] [23]. The drug maximally stimulated oxytocin secretion as early as 15 minutes after administration (5 mg/kg, IP), with effects lasting for 30 minutes [17]. This may be due to action at postsynaptic 5-HT1A receptors; somatodendritic receptors have a lower ED50 compared to those on the postsynaptic, and oxytocin stimulation was observed at high but not low doses [17]. This poses implications of how the drug may promote prosocial behaviors such as trust, which is exhibited in humans with increased levels of oxytocin [24]. Alnespirone dose dependently increased ACTH levels (at 0.01-20 mg/kg i.p), which may consequently produce downstream effects in the hypothalamic-pituitary-adrenal axis, [25] and also indirectly enhanced dopamine turnover (at 32 mg kg-1) [17]. The indirect effect of the drug on dopamine is speculated to be due to activation of 5-HT1A and subsequent 5-HT reduction that the receptor mediates [17].
Alnespirone induces dose-dependent hypothermia at 2.5-20 mg/kg in rats, but does not produce other symptoms of the 5-HT syndrome [15] .
There is also debate on Alnespirone’s effects on alcohol consumption in animal models. Studies reveal how alcohol drinking and serotonin levels share an inverse relationship, so increase in 5-HT neurotransmission results in decreased alcohol intake [12]. A significant dose-dependent decrease in alcohol intake and increased resting duration was found in adult Squirrel Monkeys treated with Alnespirone [12]. The drug also caused emesis at 0.01-0.1 mg/kg prior to the alcohol self-administration. However, results are contradictory with behavior in Long-Evans rats, in which there were significant increases in alcohol consumption after administration of Alnespirone at low doses [12]. This suggests that low doses do not potentiate the 5-HT inhibition caused by 5-HT1A autoreceptors, but may also alternatively show how 5-HT1A receptors have different effects in rats and monkeys [12].
See also[edit]
References[edit]
- ^ a b Griebel G, Misslin R, Pawlowski M, Guardiola Lemaître B, Guillaumet G, Bizot-Espiard J. (1992). "Anxiolytic-like effects of a selective 5-HT1A agonist, S20244, and its enantiomers in mice". Neuroreport. 3 (1): 84–86. doi:10.1097/00001756-199201000-00022. PMID 1351756.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Simon P, Guardiola B, Bizot-Espiard J, Schiavi P, Costentin J. (1992). "5-HT1A receptor agonists prevent in rats the yawning and penile erections induced by direct dopamine agonists". Psychopharmacology (Berl). 108 (1–2): 47–50. doi:10.1007/BF02245284. PMID 1357709. S2CID 22385029.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Astier B, Lambás Señas L, Soulière F, Schmitt P, Urbain N, Rentero N, Bert L, Denoroy L, Renaud B, Lesourd M, Muñoz C, Chouvet G. (2003). "In vivo comparison of two 5-HT1A receptors agonists alnespirone (S-20499) and buspirone on locus coeruleus neuronal activity". Eur J Pharmacol. 459 (1): 17–26. doi:10.1016/S0014-2999(02)02814-5. PMID 12505530.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "alnespirone Servier discontinued, France". R & D Focus Drug News. 6 August 2001. Retrieved 6 November 2014.
- ^ Griebel, G (1997). "Serotonergic drugs in animal models of anxiety: An update" (PDF). Serotonin ID Research Alert. 2 (6): 251–257. Retrieved 21 October 2014.
- ^ a b c d e f Kidd, EJ; Haj-Dahmane, S; Jolas, T; Lanfumey, L; Fattaccini, CM; Guardiola-Lemaitre, B; Gozlan, H; Hamon, M (Feb 1993). "New methoxy-chroman derivatives, 4[N-(5-methoxy-chroman-3-yl)N- propylamino]butyl-8-azaspiro-(4,5)-decane-7,9-dione [(+/-)-S 20244] and its enantiomers, (+)-S 20499 and (-)-S 20500, with potent agonist properties at central 5-hydroxytryptamine1A receptors". The Journal of Pharmacology and Experimental Therapeutics. 264 (2): 863–72. PMID 8094756.
- ^ Siddiqui, N; Andalip; Bawa, S; Ali, R; Afzal, O; Akhtar, MJ; Azad, B; Kumar, R (April 2011). "Antidepressant potential of nitrogen-containing heterocyclic moieties: An updated review". Journal of Pharmacy & Bioallied Sciences. 3 (2): 194–212. doi:10.4103/0975-7406.80765. PMC 3103913. PMID 21687347.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e Casanovas, JM; Vilaró, MT; Mengod, G; Artigas, F (Jan 1999). "Differential regulation of somatodendritic serotonin 5-HT1A receptors by 2-week treatments with the selective agonists alnespirone (S-20499) and 8-hydroxy-2-(Di-n-propylamino)tetralin: microdialysis and autoradiographic studies in rat brain". Journal of Neurochemistry. 72 (1): 262–72. doi:10.1046/j.1471-4159.1999.0720262.x. PMID 9886078. S2CID 13349610.
- ^ a b c d e f g Scott, PA; Chou, JM; Tang, H; Frazer, A (Jul 1994). "Differential induction of 5-HT1A-mediated responses in vivo by three chemically dissimilar 5-HT1A agonists". The Journal of Pharmacology and Experimental Therapeutics. 270 (1): 198–208. PMID 8035316.
- ^ a b c d e Casanovas, JM; Lésourd, M; Artigas, F (Oct 1997). "The effect of the selective 5-HT1A agonists alnespirone (S-20499) and 8-OH-DPAT on extracellular 5-hydroxytryptamine in different regions of rat brain". British Journal of Pharmacology. 122 (4): 733–41. doi:10.1038/sj.bjp.0701420. PMC 1564978. PMID 9375971.
- ^ a b c d e Muñoz, C; Papp, M (Aug 1999). "Alnespirone (S 20499), an agonist of 5-HT1A receptors, and imipramine have similar activity in a chronic mild stress model of depression". Pharmacology, Biochemistry, and Behavior. 63 (4): 647–53. doi:10.1016/s0091-3057(99)00031-3. PMID 10462194. S2CID 1339140.
- ^ a b c d e f McKenzie-Quirk, SD; Miczek, KA (May 2003). "5-HT1A agonists: alcohol drinking in rats and squirrel monkeys". Psychopharmacology. 167 (2): 145–52. doi:10.1007/s00213-003-1395-0. PMID 12658527. S2CID 382330.
- ^ a b Saletu, B; Grunberger, J; Anderer, P; Linzmayer, L; Semlitsch, H.V; Zyhlarz, G (June 1996). "Central effects of 5HT1A-agonists, S-20499 and buspirone: Placebo-controlled, pharmaco-EEG and psychometric studies". European Neuropsychopharmacology. 6: 203. doi:10.1016/0924-977X(96)88257-5. S2CID 54289450.
- ^ a b c d de Boer, SF; Koolhaas, JM (5 Dec 2005). "5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis". European Journal of Pharmacology. 526 (1–3): 125–39. doi:10.1016/j.ejphar.2005.09.065. PMID 16310183.
- ^ a b c d de Boer, SF; Lesourd, M; Mocaër, E; Koolhaas, JM (Jul 2000). "Somatodendritic 5-HT(1A) autoreceptors mediate the anti-aggressive actions of 5-HT(1A) receptor agonists in rats: an ethopharmacological study with S-15535, alnespirone, and WAY-100635". Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 23 (1): 20–33. doi:10.1016/S0893-133X(00)00092-0. PMID 10869883. S2CID 22824331.
- ^ a b Le Poul, E; Laaris, N; Doucet, E; Fattaccini, CM; Mocaër, E; Hamon, M; Lanfumey, L (22 Jan 1999). "Chronic alnespirone-induced desensitization of somatodendritic 5-HT1A autoreceptors in the rat dorsal raphe nucleus". European Journal of Pharmacology. 365 (2–3): 165–73. doi:10.1016/s0014-2999(98)00886-3. PMID 9988099.
- ^ a b c d e f Fabre, V; Boni, C; Mocaër, E; Lesourd, M; Hamon, M; Laporte, AM (22 Oct 1997). "[3H]Alnespirone: a novel specific radioligand of 5-HT1A receptors in the rat brain". European Journal of Pharmacology. 337 (2–3): 297–308. doi:10.1016/s0014-2999(97)01288-0. PMID 9430429. Cite error: The named reference "O" was defined multiple times with different content (see the help page).
- ^ a b c de Boer, SF; Lesourd, M; Mocaer, E; Koolhaas, JM (Mar 1999). "Selective antiaggressive effects of alnespirone in resident-intruder test are mediated via 5-hydroxytryptamine1A receptors: A comparative pharmacological study with 8-hydroxy-2-dipropylaminotetralin, ipsapirone, buspirone, eltoprazine, and WAY-100635". The Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1125–33. PMID 10027850.
- ^ a b c McGrath, C; Norman, TR (Jan 1999). "(+)-S-20499 -- a potential antidepressant? A behavioural and neurochemical investigation in the olfactory bulbectomised rat". European Neuropsychopharmacology : The Journal of the European College of Neuropsychopharmacology. 9 (1–2): 21–7. doi:10.1016/s0924-977x(97)00103-x. PMID 10082224. S2CID 21017372.
- ^ a b Porsolt, R.D; Lenegre, A; Caignard, D.H; Pfeiffer, B; Mocaer, E; Guardiola-Lemaitre, B (1992). "Psychopharmacological profile of a new chroman derivative with 5‐hydroxytryptamine1A agonist properties: S 20499 (+)". Drug Development Research. 27 (4): 389–401. doi:10.1002/ddr.430270407. S2CID 84266378.
- ^ Nathan, P.J.; Norman, T.R.; Burrows, G.D. (June 1996). "Serotonergic control of melatonin secretion: The role of 5-HT1A receptors". European Neuropsychopharmacology. 6: 80. doi:10.1016/0924-977X(96)87668-1. S2CID 54238744.
- ^ Levy, AD; Li, Q; Gustafson, M; Van de Kar, LD (14 Feb 1995). "Neuroendocrine profile of the potential anxiolytic drug S-20499". European Journal of Pharmacology. 274 (1–3): 141–9. doi:10.1016/0014-2999(94)00719-n. PMID 7768266.
- ^ Dugast, C; Soulière, F; Schmitt, P; Casanovas, JM; Fattaccini, CM; Mocaër, E; Lesourd, M; Renaud, B; Artigas, F; Hamon, M; Chouvet, G (5 Jun 1998). "Is the potent 5-HT1A receptor agonist, alnespirone (S-20499), affecting dopaminergic systems in the rat brain?". European Journal of Pharmacology. 350 (2–3): 171–80. doi:10.1016/s0014-2999(98)00254-4. PMID 9696405.
- ^ Kosfeld, M; Heinrichs, M; Zak, PJ; Fischbacher, U; Fehr, E (2 June 2005). "Oxytocin increases trust in humans". Nature. 435 (7042): 673–6. doi:10.1038/nature03701. PMID 15931222. S2CID 1234727.
- ^ Aguilera, G (December 1994). "Regulation of pituitary ACTH secretion during chronic stress". Frontiers in Neuroendocrinology. 15 (4): 321–50. doi:10.1006/frne.1994.1013. PMID 7895891. S2CID 24818312.
Category:Serotonin receptor agonists Category:Imides Category:Phenol ethers Category:Amines Category:Azapirones Category:Chromanes Category:Lactams

