Tin triphosphide

| |
| Identifiers | |
|---|---|
| Properties | |
| P3Sn | |
| Molar mass | 211.631 g·mol−1 |
| Appearance | black solid |
| Density | 4.25 g/cm3 |
| Melting point | 580 °C (1,076 °F; 853 K) |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tin triphosphide is a binary inorganic compound of tin metal and phosphorus with the chemical formula SnP3.[1]
Structure[edit]
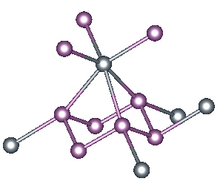
X-ray crystallography reveals that tin triphosphide is not a triphosphide. It is a hexaphosphide, with P66- rings. These ruffled P6 rings form three short (2.66 Å) and three long (2.95 Å) Sn-P bonds. The result is that Sn(II) adopts highly distorted octahedral geometry. The structure of tin triphosphide resembles that of gray arsenic, which also features corrugated, linked six-membered (As6) rings, wherein each As has a highly distorted octahedral geometry. Germanium triphosphide and tin triphosphide are similar structurally as well.
Tin triphosphide forms triclinic crystals, spatial group R3m with six formula units in a unit cell of dimensions a = 7.378 Å and c = 10.512 Å.[2][3]
Preparation and occurrence[edit]
Tin triphosphide can be formed from the fusion of stoichiometric amounts of both elements at 580 °C:
- Sn + 3P → SnP3
SnP3 has been evaluated for use in energy storage devices.[4]
Related compounds[edit]
References[edit]
- ^ Jacobson, Carl Alfred; Hampel, Clifford A. (1946). Encyclopedia of Chemical Reactions. Reinhold Publishing Corporation. p. 15. Retrieved 28 March 2024.
- ^ Gullman, Jan; Olofsson, Olle (1 November 1972). "The crystal structure of SnP3 and a note on the crystal structure of GeP3". Journal of Solid State Chemistry. 5 (3): 441–445. doi:10.1016/0022-4596(72)90091-6. ISSN 0022-4596. Retrieved 28 March 2024.
- ^ "mp-7541: SnP3 (trigonal, R-3m, 166)". Materials Project. Retrieved 28 March 2024.
- ^ Yan, Miaomiao; Yang, Bingchao; Sun, Xiujie; Wang, Zhixiu; Jiang, Xingang; Yi, Wencai; Sun, Hairui; Yang, Ruilong; Ding, Hao; Yue, Dongdong; Zhai, Kun; Li, Yueqing; Chen, Xin; Zhang, Yongsheng; Liu, Xiaobing (1 January 2024). "High-Quality 2D SnP3 Nanosheets: Novel Flexible Electrode for Energy Storage Device Application with Wide Temperature Adaptivity". ACS Materials Letters. 6 (1): 194–202. doi:10.1021/acsmaterialslett.3c01438. ISSN 2639-4979. Retrieved 28 March 2024.
- ^ Donohue, Paul C. (1970). "Synthesis, Structure, and Superconducting Properties of New High-Pressure Forms of Tin Phosphide". Inorganic Chemistry. 9 (2): 335–337. doi:10.1021/ic50084a032.
- ^ Olofsson, Olle; Jerslev, Bodil; Thom, Erling; Stoll, E.; Eriksson, G.; Blinc, R.; Paušak, S.; Ehrenberg, L.; Dumanović, J. (1967). "On the Crystal Structure of Sn4P3". Acta Chemica Scandinavica. 21: 1659–1660. doi:10.3891/acta.chem.scand.21-1659.
