Talk:Amphetamine/Archive 8
| This is an archive of past discussions. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 5 | Archive 6 | Archive 7 | Archive 8 |
Evidence vs. usage
In my view, the most problematic part of the Amphetamine article is that it overemphasizes "amphetamine is used for" and underemphasizes "there is evidence of efficacy and safety of amphetamine for treatment of." Both evidence and usage ought to be described (of course, this is an encyclopedia, after all), but there should be balance. Some readers may infer from the language in the first sentence of the lede that it is reasonable to treat obesity with amphetamine, however I expect that most experts would consider the harms of using amphetamines for obesity to outweigh the benefits. The lede could cite a recent large, well-done study of prevalence of use.[1] It's not a meta-analysis, but is the best quality evidence we are likely to get.
Here's a draft sentence to consider for the lede: A 2018 study estimated that approximately 16 million people in the United States take amphetamines.[1]
Please note that the AACE/ACE guidelines for obesity treatment do not recommend amphetamine nor do they even recommend phentermine alone, and the guidelines caution prescribers about use of lorcaserin (which can be abused by using high doses). (The extended-release combo of phentermine/topiramate is on the AACE/ACE list of recommended drugs.) this reflects a consensus among experts about use of drugs with abuse potential for treatment of obesity. [2]
Sbelknap (talk) 22:09, 19 July 2018 (UTC)
- Your concern is actually very similar to the one I mentioned at WT:MED. This article really only covers the safety/efficacy of amphetamine for ADHD, but it should cover this for ADHD, obesity, and narcolepsy. Unfortunately, there really aren't any meta-analyses that cover the efficacy of amphetamine for obesity (see this search). I would like to create the subsections on narcolepsy and obesity sometime in the near future though.
- As for the lead, it wouldn't be a good idea to cover the safety/efficacy of its uses there since this article needs to give comparable coverage to each major subtopic in a very small amount of space (the limit for the size of the lead is 4 paragraphs for very large articles, per MOS:LEADLENGTH). I understand your desire to cover these things in the lead since many people come to articles and read leads, but I don't think that's entirely true for drug articles; e.g., whenever I go to an article as a reader and not an editor, I often ignore the lead altogether and go to the section pertaining to the topic that I'm interested in (and sometimes end up searching pubmed for relevant articles and expanding that section if I found it lacking). I can't imagine someone interested in knowing about its medical uses or pharmacology would stop at the 4 introductory paragraphs that summarize an entire article (especially one that's as massive as this one).
So, in a nutshell, I think the best approach would be to create the sections on obesity and narcolepsy. First, we need to get sources on amphetamine's efficacy for those uses though (i.e., meta-analyses for narcolepsy and medical reviews for obesity). I'll probably have time to look for sources on efficacy tonight once I've finished fleshing out the medical uses section of bupropion with the meta-analyses that I cited on bupropion's talk page. Once we have those sources, we can write those sections of this article. - As for the study you provided, my main concerns with using it to indicate the prevalence of prescription amphetamine use is that it doesn't quantify the use of amphetamine alone (i.e., it includes other prescription stimulants, like methylphenidate - which isn't a substituted amphetamine - and methamphetamine) and articles should ideally provide a global perspective. The global prevalence of both the licit (i.e., prescription) and illicit use of amphetamine-type stimulants is actually included in this article at present though: see the 1st table under Amphetamine#History, society, and culture. Seppi333 (Insert 2¢) 01:54, 20 July 2018 (UTC)
Obesity
I ran this search for recent reviews/practice guidelines/meta-analyses/systematic reviews that include amphetamine (in the title/abstract or mesh terms), obesity (in the title/abstract), and efficacy (in the title/abstract); came up with 7 sources and all of them were irrelevant. After repeating that search without the efficacy term, I found ~40. The only one that actually covered the efficacy of amphetamine for weight loss examined really old trials: PMID 25194183 doi:10.1016/j.bpg.2014.07.015). I don't think we'd be able to cover the efficacy in the obesity section. The only things we probably can cover are: (1) amphetamine is intended to be used as an adjunct therapy for a period of several weeks (per the prescribing information[3]) and (2) what non-Pubmed indexed practice guidelines say about it. I don't think it's worth covering the mechanism of action for this given the lack of evidence on efficacy. Seppi333 (Insert 2¢) 12:41, 20 July 2018 (UTC)
List of references relevant to this section:
Narcolepsy
I'm running into a similar problem with sources on efficacy for narcolepsy. There's a lot of reviews, but no pubmed indexed meta-analyses, practice guidelines, or systematic reviews that actually quantify its efficacy on the basis of some metric of symptom reduction. The main difference is that all of the reviews I've read in the past and skimmed through now all assert that it has treatment efficacy. I'll probably end up citing clinical practice guidelines for narcolepsy as well. These sources seem relevant: AASM guideline, AASM review, PMID 29759269, 26716917, 27549768, 28424564.
- The AASM narcolepsy review states the following about the efficacy/use of amphetamine:
Selected excerpts from the AASM narcolepsy review
|
|---|
|
- The AASM narcolepsy guideline states the following about amphetamine:
Selected excerpts from the AASM narcolepsy guideline
|
|---|
|
- PMID 29759269 (doi:10.1016/j.jsmc.2018.02.009) mentions:
|
- Random aside: this review also asserted something I thought was interesting:
the alerting effect of 6 cups of strong coffee is comparable with that of 5 mg of dexamphetamine.
- Random aside: this review also asserted something I thought was interesting:
- PMID 26716917 (doi:10.1056/NEJMra1500587) mentions:
Methylphenidate, as well as dextroamphetamine and similar amphetamines, can be more potent than modafinil, but side effects are more common with these drugs.
It doesn't really go that much into treatment efficacy. Figure 2A is a pretty good diagram of the ascending reticular activating system though. - PMID 27549768 – still need to read through this review.
- PMID 28424564 – still need to read through this review.
I need to read through the last 2 reviews and check for additional practice guidelines for narcolepsy. I don't have any more time to do this right now, so I'll continue this later. Seppi333 (Insert 2¢) 00:11, 21 July 2018 (UTC)
Section reflist
References
- ^ a b Compton WM, Han B, Blanco C, Johnson K, Jones CM (April 2018). "Prevalence and Correlates of Prescription Stimulant Use, Misuse, Use Disorders, and Motivations for Misuse Among Adults in the United States". Am J Psychiatry. 175 (8): 741–755. doi:10.1176/appi.ajp.2018.17091048. PMC 6070393. PMID 29656665.
- ^ http://obesity.aace.com/files/obesity/guidelines/aace_guidelines_obesity_2016.pdf
- ^ "Evekeo Prescribing Information" (PDF). Arbor Pharmaceuticals LLC. September 2016. pp. 1–2. Retrieved 20 July 2018.
References tweaked
I removed pointers/links to sci-hub, as links to that site are blacklisted on en.wp and the WP:TLD keeps changing anyway, instead leaving DOI links. Obviously if you want to use sci-hub, that is enough info to find the articles there. DMacks (talk) 12:24, 17 October 2018 (UTC)
Updates
Monoaminergic/glutamatergic mechanisms
Things to add once I come across a second review covering all of these together - needs to provide context (citations for each are in the collapse tab below):
- Cover RhoA+ROCK-mediated DAT internalization - add to Amphetamine#Dopamine
- Cover RhoA-mediated EAAT3 internalization in mesolimbic dopamine neurons - add to Amphetamine#Other neurotransmitters, peptides, and hormones after the current sentence on EAAT3.
- Mention that VGlut2 (SLC17A6) is expressed in mesolimbic dopamine neurons and that both dopamine and glutamate are released from mesolimbic dopamine neurons - add to Amphetamine#Other neurotransmitters, peptides, and hormones immediately following the content on amphetamine-induced EAAT3 internalization.
Seppi333 (Insert 2¢) 21:11, 6 December 2016 (UTC)
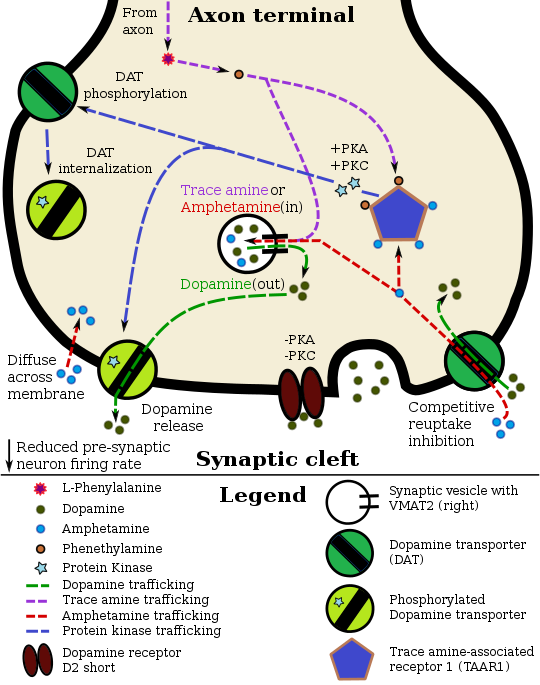
Pharmacodynamics diagram updates + new pharmacodynamics table
| |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CAMKII signalingCheck for newer research involving:
Seppi333 (Insert 2¢) 02:00, 31 March 2016 (UTC) Updated 02:05, 27 April 2016 (UTC) RhoA/ROCK signaling + DAT/EAAT3 internalization
Seppi333 (Insert 2¢) 22:02, 6 November 2016 (UTC); Updated 17:53, 14 November 2016 (UTC) Wikitable to add when updating pharmacodynamics diagramWill
The phosphorylation / inactivation of RhoA by PKA occurs roughly 10–15 minutes following neuronal exposure to amphetamine and more or less plateaus by 20–30 minutes post-exposure, based upon in vitro research. Seppi333 (Insert 2¢) 17:53, 14 November 2016 (UTC) Amphetamine-induced ERK1/2-mediated phosphorylation of DAT on the Thr53 residue, which induces DA efflux, also appears to occur;[2][3] ERK1/2 is likely activated by the PKCβ isoform of PKC.[3]
Tentative changes to diagram
Things to add to or change in the
† need to wait for a ref to be published which explicitly states that DAT and EAAT3 are phosphorylated by a ROCK or a different RhoA-activated protein kinase
| |||||||||||||||||||||||||||||||||||||||||||||||||
Addiction
- Really interesting papers based upon the abstracts; probably useful information worth adding to relevant articles: PMID 31749652, 29511807, 31071288, 31698743, 31680830
- Need to indicate that the current evidence base for physical exercise as a treatment for amph/meth addiction supports its use even as a monotherapy sans behavioral therapy and is more effective than CBT in certain outcome domains. Also a stronger evidence base for exercise as an addiction prophylactic PMID 31344831, 29266758, 29430384, 29338767
- PMID 31695630 - interesting cognitive control-based strategy (maximal utilization of working memory) to interfere with craving/incentive salience for drug use
- May 2017 meta-analysis[1]
References
- ^ Ashok AH, Mizuno Y, Volkow ND, Howes OD (May 2017). "Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-analysis". JAMA Psychiatry. 74 (5): 511–519. doi:10.1001/jamapsychiatry.2017.0135. PMC 5419581. PMID 28297025.
Menstrual cycle
- Need more evidence on this before covering sex-dependent differences in drug response. Seppi333 (Insert 2¢) 17:39, 25 December 2019 (UTC)
References
- ^ Van Voorhees EE, Mitchell JT, McClernon FJ, Beckham JC, Kollins SH (May 2012). "Sex, ADHD symptoms, and smoking outcomes: an integrative model". Med. Hypotheses. 78 (5): 585–593. doi:10.1016/j.mehy.2012.01.034. PMC 3321070. PMID 22341778.
research with cocaine and amphetamine in humans has found that the women report greater positive subjective effects of both substances during the follicular than the luteal phase of the menstrual cycle [129]. Moreover, men report greater positive subjective effects of stimulants compared to women who are in the luteal phase, though these gender differences disappear during the follicular phase [104, 130, 131]. Some [130, 131] but not all [132] research has found plasma or salivary estrogen levels to be associated positively with subjective response to amphetamine, and one study found that exogenously administered estrogen enhanced the discriminative stimulus effects of low doses of amphetamine [106].
Effects
- [1] - may be worth covering some content from this chapter:
Seppi333 (Insert 2¢) 08:28, 4 December 2015 (UTC)
- Updated 06:54, 9 October 2016 (UTC)
- Updated 17:39, 25 December 2019 (UTC)
References
- ^ Gunne LM (2013). "Effects of Amphetamines in Humans". Drug Addiction II: Amphetamine, Psychotogen, and Marihuana Dependence. Berlin, Germany; Heidelberg, Germany: Springer. pp. 247–260. ISBN 9783642667091. Retrieved 4 December 2015.
- Sooo, it's literally just synthetic adrenaline? lol 67.193.213.188 (talk) —Preceding undated comment added 16:58, 24 June 2020 (UTC)
Completed updates
Older addiction content
- Review: human studies involving environmental enrichment and physical exercise[1]
 Partly done - need to cover research on human environmental enrichment-based addiction treatments in Amphetamine#Behavioral treatments and summarize the added material in the 2nd paragraph under Amphetamine#Overdose. Seppi333 (Insert 2¢) 21:30, 16 November 2016 (UTC)
Partly done - need to cover research on human environmental enrichment-based addiction treatments in Amphetamine#Behavioral treatments and summarize the added material in the 2nd paragraph under Amphetamine#Overdose. Seppi333 (Insert 2¢) 21:30, 16 November 2016 (UTC)
References
- ^ Carroll ME, Smethells JR (February 2016). "Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments". Front. Psychiatry. 6: 175. doi:10.3389/fpsyt.2015.00175. PMC 4745113. PMID 26903885.
Environmental Enrichment ...
In humans, non-drug rewards delivered in a contingency management (CM) format successfully reduced drug dependence [for a review see Ref. (188)]. In general, CM programs promote drug abstinence through a combination of positive reinforcement for drug-free urine samples. For instance, voucher-based reinforcement therapy in which medication compliance, therapy session attendance, and negative drug screenings reinforced with vouchers to local business (e.g., movie theater, restaurants, etc.) directly reinforces drug abstinence, provides competing reinforcers, enriches the environment, and it is a robust treatment across a broad range of abused drugs (189). ...
Physical Exercise
There is accelerating evidence that physical exercise is a useful treatment for preventing and reducing drug addiction [see reviews in Ref. (28, 178, 190, 191)]. In some individuals, exercise has its own rewarding effects, and a behavioral economic interaction may occur, such that physical and social rewards of exercise can substitute for the rewarding effects of drug abuse. ... The value of this form of treatment for drug addiction in laboratory animals and humans is that exercise, if it can substitute for the rewarding effects of drugs, could be self-maintained over an extended period of time. Work to date in laboratory animals [for review, see Ref. (191)] and humans [for review, see Ref. (178)] regarding exercise as a treatment for drug addiction supports this hypothesis. ... However, a RTC study was recently reported by Rawson et al. (226), whereby they used 8 weeks of exercise as a post-residential treatment for METH addiction, showed a significant reduction in use (confirmed by urine screens) in participants who had been using meth 18 days or less a month. ... Animal and human research on physical exercise as a treatment for stimulant addiction indicates that this is one of the most promising treatments on the horizon. [emphasis added]{{cite journal}}: CS1 maint: unflagged free DOI (link)
- [1] - also need to update table entry
 Done
Done
Seppi333 (Insert 2¢) 17:33, 16 November 2015 (UTC)
References
Abuse contraindication
- Add note on abuse history and prescriptions[1]
 Done
Done
Seppi333 (Insert 2¢) 21:21, 9 December 2015 (UTC)
References
- ^ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
In reality, there is little abuse of these drugs by patients with ADHD (Merkel and Kuchibhatla, 2009), and in most cases the challenge for the prescribing doctor is to keep the patients taking their medication rather than limiting its use. Many teenage patients stop using despite the drugs having clear benefits for their school performance; they cite reasons such as feeling too controlled, wanting empowerment from medication, etc. For these reasons, observations of dependence and abuse of prescription d-amphetamine are rare in clinical practice, and this stimulant can even be prescribed to people with a history of drug abuse provided certain controls, such as daily pick-ups of prescriptions, are put in place (Jasinski and Krishnan, 2009b).
{{cite journal}}: CS1 maint: multiple names: authors list (link)
another ergogenic effect
- greater power output without any change in perceived exertion[1]
 Added
Added - neurophysiological mechanism is mediated by CNS dopamine, which allows the body to increase power output without affecting perceived exertion, presumably by raising the core temperature limit.[2]
 Added
Added
Seppi333 (Insert 2¢) 08:18, 9 March 2016 (UTC)
References
- ^ Rattray B, Argus C, Martin K, Northey J, Driller M (March 2015). "Is it time to turn our attention toward central mechanisms for post-exertional recovery strategies and performance?". Front. Physiol. 6: 79. doi:10.3389/fphys.2015.00079. PMC 4362407. PMID 25852568.
Aside from accounting for the reduced performance of mentally fatigued participants, this model rationalizes the reduced RPE and hence improved cycling time trial performance of athletes using a glucose mouthwash (Chambers et al., 2009) and the greater power output during a RPE matched cycling time trial following amphetamine ingestion (Swart, 2009). ... Dopamine stimulating drugs are known to enhance aspects of exercise performance (Roelands et al., 2008)
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Roelands B, De Pauw K, Meeusen R (June 2015). "Neurophysiological effects of exercise in the heat". Scand. J. Med. Sci. Sports. 25 Suppl 1: 65–78. doi:10.1111/sms.12350. PMID 25943657.
Physical fatigue has classically been attributed to peripheral factors within the muscle (Fitts, 1996), the depletion of muscle glycogen (Bergstrom & Hultman, 1967) or increased cardiovascular, metabolic, and thermoregulatory strain (Abbiss & Laursen, 2005; Meeusen et al., 2006b). In recent decennia however, it became clear that the central nervous system plays an important role in the onset of fatigue during prolonged exercise (Klass et al., 2008), certainly when ambient temperature is increased ... 5-HT, DA, and NA have all been implicated in the control of thermoregulation and are thought to mediate thermoregulatory responses, certainly since their neurons innervate the hypothalamus (Roelands & Meeusen, 2010). ... Strikingly, both the ratings of perceived exertion and the thermal sensation were not different to the placebo trial. This indicates that subjects did not feel they were producing more power and consequently more heat. ... Taken together, these data indicate strong ergogenic effects of an increased DA concentration in the brain, without any change in the perception of effort. ... The combined effects of DA and NA on performance in the heat were studied by our research group on a number of occasions. ... the administration of bupropion (DA/NA reuptake inhibitor) significantly improved performance. Coinciding with this ergogenic effect, the authors observed core temperatures that were much higher compared with the placebo situation. Interestingly, this occurred without any change in the subjective feelings of thermal sensation or perceived exertion. Similar to the methylphenidate study (Roelands et al., 2008b), bupropion may dampen or override inhibitory signals arising from the central nervous system to cease exercise because of hyperthermia, and enable an individual to continue maintaining a high power output
Ntox
- Review - covers relationship between amphetamine-induced hyperthermia and neurotoxicity[1]
 Added
Added
Seppi333 (Insert 2¢) 22:02, 6 November 2016 (UTC)
References
- ^ Bowyer JF, Hanig JP (November 2014). "Amphetamine- and methamphetamine-induced hyperthermia: Implications of the effects produced in brain vasculature and peripheral organs to forebrain neurotoxicity". Temperature (Austin). 1 (3): 172–182. doi:10.4161/23328940.2014.982049. PMC 5008711. PMID 27626044.
Hyperthermia alone does not produce amphetamine-like neurotoxicity but AMPH and METH exposures that do not produce hyperthermia (≥40°C) are minimally neurotoxic. Hyperthermia likely enhances AMPH and METH neurotoxicity directly through disruption of protein function, ion channels and enhanced ROS production. Forebrain neurotoxicity can also be indirectly influenced through the effects of AMPH- and METH- induced hyperthermia on vasculature. The hyperthermia and the hypertension produced by high doses amphetamines are a primary cause of transient breakdowns in the blood-brain barrier (BBB) resulting in concomitant regional neurodegeneration and neuroinflammation in laboratory animals. ... In animal models that evaluate the neurotoxicity of AMPH and METH, it is quite clear that hyperthermia is one of the essential components necessary for the production of histological signs of dopamine terminal damage and neurodegeneration in cortex, striatum, thalamus and hippocampus.
Carbonic anhydrases
Brain carbonic anhydrase activation
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
@Boghog: Can you help me create CA5A/carbonic anhydrase 5A and CA13/carbonic anhydrase 13? Those are the only carbonic anhydrase genes/proteins that WP is missing articles on (according to list of human protein-coding genes 1). Seppi333 (Insert 2¢) 22:19, 31 December 2019 (UTC)
References
|
Re
@Doc James and Casliber: Would like your feedback on Amphetamine#Pharmacomicrobiomics; pinging you two since you recently took an interest in this article. Only the second paragraph is technically "on topic" and within the article's scope, but the 2nd paragraph almost seems like trivia without a contextual understanding, which is what the first paragraph provides: it states the types of clinically significant drug-microbiota interactions. Really just looking for general feedback, but also wondering whether you think this particular statement from the 1st paragraph is worth keeping or cutting: The nascent and active field of research on drug-microbe interactions – known as pharmacomicrobiomics – lies at the intersection of systems microbiology, genomics, systems pharmacology, and personalized medicine.
@AmericanLemming: If you could proofread/copyedit this section like you did with the other technical ones during the FAC review, I'd greatly appreciate it. ![]() I did my best to make it accessible, but I think you might be a better judge of what is/isn't understandable to a layperson than me for this topic. Seppi333 (Insert 2¢) 16:37, 5 August 2019 (UTC)
I did my best to make it accessible, but I think you might be a better judge of what is/isn't understandable to a layperson than me for this topic. Seppi333 (Insert 2¢) 16:37, 5 August 2019 (UTC)
- I agree that the first paragraph is too off-topic to include. I'd remove it. Cas Liber (talk · contribs) 20:34, 5 August 2019 (UTC)
Initial comments
- 1. I agree with CasLiber that the first paragraph is a bit off-topic; the information there probably fits better in the main pharmacomicrobiomics article.
- 2. Also, it would be helpful to briefly explain the significance/implications of this study; for example, do the authors think that differences in the microbiome between individuals might explain some of the differences in how they respond to amphetamine?
- 3. I noticed that the link for tyramine oxidase redirects to the article on monoamine oxidase; is this bacterial enzyme analogous to human monoamine oxidase?
That's all for today. AmericanLemming (talk) 01:05, 7 August 2019 (UTC)
- Re 1&2: Excluding the statements in the note and the first sentence about microbial and human genomes, everything in the first paragraph is mentioned in that study; the clinical significance of the finding is also mentioned in the paper, but I figured that since it's a primary source, I probably shouldn't be using it to cite a clinical claim. In a nutshell, the clinical significance is that E. coli can affect amphetamine's pharmacokinetics (its oral bioavailability and its metabolism) and variations in gastrointestinal E. coli colonization/concentration between individuals explains some of the inter-individual variation in amphetamine's clinical response (very high prevalence of E. coli in the gut → reduced clinical efficacy relative to people with normal [low] amounts of E. coli in the gut).[1] It goes on to discuss the use of the findings in redesigning the drug (see the quote below or read the end of the paper here:
sci-hub.tw/10.1002/jcb.28396); however, I don't think the authors knew that amphetamine's prodrug (lisdexamfetamine) isn't converted into dextroamphetamine until it's absorbed into human blood plasma, which is (normally) sterile. I don't think it's likely that lisdexamfetamine would be metabolized by E. coli's tyramine oxidase due to the sizable difference in the chemical structure, but I may be wrong. @Doc James: do you think I should just cite the paper for the clinical statements? - Re 3: the tyramine oxidase they're referring to is the tyramine oxidase encoded by E. coli's tynA gene (this isn't mentioned in the paper, but they refer readers to expasy
.org, which after navigating through the cross-links, one can find that tynA is listed as the encoding gene for E. coli's tyramine oxidase; also, the tyramine oxidase for one of the E. coli strains (i.e., MS 116‐1) that were mentioned in the paper (strains ATCC 8739, HS, MS 116‐1, MS 146‐1, MS 175‐1) on NCBI Protein is listed as being encoded by tynA). The correct article on this would be primary amine oxidase (per the NCBI Protein link, [1], and the UniProt entry for tynA in E. coli) since that's the name of the protein that tynA encodes and the fact that we normally use protein names as article titles. - Tangential point: Based upon the reaction that tyramine oxidase catalyzes (i.e., RCH2NH2 + H2O + O2 RCHO + NH3 + H2O2) and more specifically the fact that tynA metabolizes phenethylamine into phenylacetaldehyde and tyramine (4-hydroxyphenethylamine) into 4-hydroxyphenylacetaldehyde (I created this article today due to the number of red backlinks; the metabolic pathway I'm talking about here is now covered in that article), E. coli would seem to metabolize amphetamine into alpha-methylphenylacetaldehyde (which is probably further metabolized by E. coli's feaB enzyme into a methylated derivative of phenylacetate, based upon the metabolic fate of phenylacetaldehye and 4-hydroxyphenylacetaldehyde in E. coli - this pathway is covered in the UniProt link); that compound isn't naturally produced by any human enzymes. I obviously can't state any of this in the article since I can't (yet) cite a paper that explicitly covers it. Seppi333 (Insert 2¢) 11:31, 7 August 2019 (UTC)
- Edit: I fixed the tyramine oxidase link in the article and mentioned tyramine oxidase/tynA in primary amine oxidase. The tyramine oxidase redirect is probably correctly targeted since I'm fairly certain that synonym maps either to the human MAOA gene or both human MAO genes. Seppi333 (Insert 2¢) 05:16, 8 August 2019 (UTC)
- Is this clinically important. Doc James (talk · contribs · email) 13:55, 8 August 2019 (UTC)
- Yes, and this information can be used clinically in any doctors' office that can test for microbial concentrations of specific strains of bacteria (which admittedly is very few at the moment, as it would require employing the same molecular biology techniques as required for diagnosing small intestinal bacterial overgrowth - i.e., gut fluid aspiration and a microbiological culture). Seppi333 (Insert 2¢) 03:58, 10 August 2019 (UTC)
- Is this clinically important. Doc James (talk · contribs · email) 13:55, 8 August 2019 (UTC)
- Re 1&2: Excluding the statements in the note and the first sentence about microbial and human genomes, everything in the first paragraph is mentioned in that study; the clinical significance of the finding is also mentioned in the paper, but I figured that since it's a primary source, I probably shouldn't be using it to cite a clinical claim. In a nutshell, the clinical significance is that E. coli can affect amphetamine's pharmacokinetics (its oral bioavailability and its metabolism) and variations in gastrointestinal E. coli colonization/concentration between individuals explains some of the inter-individual variation in amphetamine's clinical response (very high prevalence of E. coli in the gut → reduced clinical efficacy relative to people with normal [low] amounts of E. coli in the gut).[1] It goes on to discuss the use of the findings in redesigning the drug (see the quote below or read the end of the paper here:
References
- ^ Kumar K, Dhoke GV, Sharma AK, Jaiswal SK, Sharma VK (January 2019). "Mechanistic elucidation of amphetamine metabolism by tyramine oxidase from human gut microbiota using molecular dynamics simulations". Journal of Cellular Biochemistry. 120 (7): 11206–11215. doi:10.1002/jcb.28396. PMID 30701587.
Numerous microorganisms reside with the human host in a symbiotic relationship and play an important role in the host metabolic processes and health.1,2 Several studies in the recent past have reported that there are compositional differences in the human microbiome due to factors such as geographical location, diet, age, and genetic variations.3 Particularly in the case of the human gut, which harbors a large diversity of bacterial species, the differences in microbial composition can significantly alter the metabolic activity in the gut lumen.4 The differential metabolic activity due to the differences in gut microbial species has been recently linked with various metabolic disorders and diseases.5-12 In addition to the impact of gut microbial diversity or dysbiosis in various human diseases, there is an increasing amount of evidence which shows that the gut microbes can affect the bioavailability and efficacy of various orally administrated drug molecules through promiscuous enzymatic metabolism.13,14 ... The present study on the atomistic details of amphetamine binding and binding affinity to the tyramine oxidase along with the comparison with two natural substrates of this enzyme namely tyramine and phenylalanine provides strong evidence for the promiscuity‐based metabolism of amphetamine by the tyramine oxidase enzyme of E. coli. The obtained results will be crucial in designing a surrogate molecule for amphetamine that can help either in improving the efficacy and bioavailability of the amphetamine drug via competitive inhibition or in redesigning the drug for better pharmacological effects. This study will also have useful clinical implications in reducing the gut microbiota caused variation in the drug response among different populations.
Follow-up comments
I’m pretty satisfied with the section as a whole; I just have some suggestions for replacing some technical language with simpler language that conveys largely the same meaning.
- 4. In the first paragraph, it says “Homo sapiens cells”; I would just call them “human cells”.
- 5. Also, I think it would still be a good idea to give a brief definition of what “pharmacomicrobiomics” means and link to the article on it. I suggest something like “there is considerable potential for interactions between drugs and an individual's microbiome. The study of these interactions is called pharmacomicrobiomics, which include drugs altering the composition of the human microbiome…”
- 6. As for the second paragraph, we should probably link promiscuous metabolism (needs to be piped) and include a brief definition in a footnote, since I don't feel that the introduction to the Enzyme promiscuity article is all that helpful for a non-expert like me.
- 7. Replacing human gastrointestinal microbiota with “gut bacteria” would probably be an oversimplification, but if bacteria are mainly responsible for drug metabolism by microbes, could we put say “primarily bacteria” in parentheses afterward?
- 8. Also, replacing “into blood plasma” with “into the blood” would sacrifice a little precision but would be a little simpler. AmericanLemming (talk) 03:03, 13 August 2019 (UTC)
- @AmericanLemming: Sorry about the long delay in my reply; I'd meant to respond earlier but was tied up at the time and forgot about it in the meantime.
- 4.
 Done
Done - 5.
 Added at the end of the first paragraph: "The field that studies these interactions is known as pharmacomicrobiomics."
Added at the end of the first paragraph: "The field that studies these interactions is known as pharmacomicrobiomics." - 6. I think what the authors meant here was that amphetamine is likely a ligand for (i.e., likely metabolized by) many currently unidentified microbial enzymes, not that the enzymes themselves are promiscuous.
- 7.
 Done
Done - 8.
 Done - changed it to "into the blood stream"
Done - changed it to "into the blood stream" - Seppi333 (Insert 2¢) 12:07, 16 August 2019 (UTC)
@AmericanLemming: ![]() Thank you for reviewing that for me! Seppi333 (Insert 2¢) 06:34, 18 August 2019 (UTC)
Thank you for reviewing that for me! Seppi333 (Insert 2¢) 06:34, 18 August 2019 (UTC)
I find this page to be biased in the emphasis on how safe amphetamines are and that when abused then there are problems. Many people end up with issues at prescribed doses such as withdrawal and dependence which can also be found in research. Mentions how younger kids are less likely to abuse drugs later on in life if they start on amphetamines at a young age. What is skipped is that starting at any other age increases the chance. Ignores acute tolerance which happens within hours of taking the medication and was used to base the design of various extended release products. Ignores many harmful aspects such as oxidative stress, downregulation of receptors and the production of neurotransmitters, neurotransmitter depletion, Instead it only mentions pathways to addiction. Dependence is related, but not mentioned when the brain no longer efficiently manages it's own neurotransmitters and has to rely on the medication to do it. Even at low doses people often feel the Adderall crash when it wears off. All be it mildly. Feels like every section has to say something about it's safe at prescribed doses or implies negative outcomes only for abusers. Seems more like damage control and people trying to sell me on the idea. Safety and what not should be in one section, not in every section as if trying to protect peoples perceptions. Not a single mention of the effects on the endocrine system. Skipped the issue on stunted growth all together. etc. Aside from that, very informative. — Preceding unsigned comment added by 69.248.160.198 (talk) 10:32, 9 April 2023 (UTC)
- I don't 🤷 Strawkipedia (talk) 06:29, 8 July 2023 (UTC)
Mentions how younger kids are less likely to abuse drugs later on in life if they start on amphetamines at a young age. What is skipped is that starting at any other age increases the chance.
That's news to me. Feel free to cite a reliable medical source, and I'd add that information myself.Many people end up with issues at prescribed doses such as withdrawal and dependence which can also be found in research.
Yes, it's mild and lasts about a week, if it occurs at all. It's not mild for recreational users, but it still only persists for a few weeks even in the heaviest recreational users. Why does this deserve to be mentioned for people taking therapeutic doses when it's generally subclinical (i.e., doctors and psychiatrists discontinuing the medication don't gradually taper the dose), if it occurs at all, though? Moreover, wouldn't covering this reaffirm your belief that the article is biased in convincing you of safety?Ignores acute tolerance which happens within hours of taking the medication and was used to base the design of various extended release products.
What?Ignores many harmful aspects such as oxidative stress, downregulation of receptors and the production of neurotransmitters, neurotransmitter depletion,
Feel free to cite some medical sources discussing evidence found in humans, and I'll add it to the article.Skipped the issue on stunted growth all together.
You clearly did not actually read the article. It's been covered in Amphetamine#Contraindications for at least the past 6 years.- Generally speaking, for a drug that's used clinically and recreationally, why do you expect the article to conflate the effects of clinical low-dose use and recreational high-dose use? It either biases the reader into thinking recreational use is safer than it actually is, clinical use is less safe than it actually is, or both. I don't particularly care whether the inclusion of content makes people think the article is biased. I do care about the omission of content, though.
WP:FACR: 2c
@Headbomb: You updated 2 page ranges, added 2 pmcs, added 3 issues, and added a missing year in [2]; those were the useful revisions. Meanwhile, you changed some of the abbreviated journal titles to full titles, while leaving e.g., references 101, 102, 103, and 105 abbreviated. All of the journals were consistently formatted before you used a bot to bork the citation formatting (which is why those bots were denied from editing this page before you removed them from the template). You need to consistently format the journal titles in this article or I'm going to revert your edit. It's not my job to clean up after bot edits that inconsistently revise this page's citations. Seppi333 (Insert 2¢) 20:06, 24 December 2019 (UTC)
- Should be fixed now. Citations were located in different templates. Headbomb {t · c · p · b} 20:17, 24 December 2019 (UTC)
Abbreviations
@Seppi333: It isn't clear if MOS:FIRSTABBR should apply to citations since they stand alone in their usage. For example, there is no problem with repeating the same link in many citations within an article MOS:REPEATLINK. Whywhenwhohow (talk) 06:22, 25 December 2019 (UTC)
- @Whywhenwhohow: I'm familiar with the exception for links in citations; I simply dislike them. When I click something in a citation, I expect to navigate outside WP, not internally. Ignoring the guideline entirely, an abbreviation for a website or publisher entry is just extraneous markup; it doesn't serve any purpose like it does in the article text.
- Anyway, can you restore the publisher parameters in the citations to the FDA website that you changed to DailyMed? It should be listed as the manufacturer; regardless of what website hosts the prescribing information, the publisher would still remain the same. I already restored a few, but there's others that need to be fixed. Seppi333 (Insert 2¢) 06:58, 25 December 2019 (UTC)
- Yes, but why do you prefer the ones from the FDA website? They are PDF format and harder to navigate? The NIH DailyMed website is the official provider of FDA label information Whywhenwhohow (talk) 08:27, 25 December 2019 (UTC)
- I don't have a fixed preference for DailyMed or the FDA's labels as a citation since both have an advantage over the other; the actual content is identical though. The PDFs have page numbers which makes verifiability easier when the prescribing information is rather long, but DailyMed is easier to navigate given its design. I'm just used to citing FDA labels since I've always used Drugs@FDA to search for drug information (e.g., the label itself, approval data, and information on current/actively marketed brands as well as discontinued drug products associated with the active ingredient I'm searching).
- In any event, I just realized that I worded my earlier request about the publisher information very poorly; what I meant to say was to restore the publisher parameters from the FDA citations in the DailyMed citations. Databases like Drugs@FDA and DailyMed that host a drug label online should always just be listed in the website/work parameter of
{{cite web}}, so you were doing that part correctly. The publishers are the entities that write/edit and produce a document, which in this case is the prescribing information − i.e., pharmaceutical companies like Shire Plc, Hoffmann-La Roche, Merck & Co, etc.. While the FDA approves prescribing information for drugs, the drug label for any given drug is the copyrighted intellectual property of the manufacturer/pharmaceutical company that produces the drug because they're the authors of the corresponding drug label; that's why they're listed as the publisher for drug labels. I've added the original citation templates below as an example. In any event, I'll go ahead and fix the DailyMed citations since you already spent time actioning my poorly worded request. Seppi333 (Insert 2¢) 09:59, 25 December 2019 (UTC) - They're all fixed now. If you want to change the 2 FDA drug label citations I added to DailyMed citations, feel free to do so. I don't really care which one we use. Seppi333 (Insert 2¢) 10:19, 25 December 2019 (UTC)
- Yes, but why do you prefer the ones from the FDA website? They are PDF format and harder to navigate? The NIH DailyMed website is the official provider of FDA label information Whywhenwhohow (talk) 08:27, 25 December 2019 (UTC)
Drug label citation templates
|
|---|
|
Page size
At 241,890 bytes of wiki markup, this page is far too large. That's particularly troubling for a page listed as a featured article. How can it best be divided? For example, splitting off the entire 'Pharmacology' section would remove over a fifth of that (less if a summary is kept here). Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 11:19, 2 January 2020 (UTC) @Pigsonthewing:
- Document statistics:
- File size: 989 kB
- Prose size (including all HTML code): 123 kB
- References (including all HTML code): 25 kB
- Wiki text: 236 kB
- Prose size (text only): 49 kB (6811 words) "readable prose size"
- References (text only): 2506 B
- User_talk:Dr_pda/prosesize.js
- To quote WP:TOOBIG:
These rules of thumb apply only to readable prose and not to wiki markup size (as found on history lists or other means), and each kB can be equated to 1,000 characters.
- I'm not even going to consider splitting this article right now given that it's not even that long by the actual guideline metric. Seppi333 (Insert 2¢) 11:43, 2 January 2020 (UTC)
- Wow, four bigs, and and underline? -- Mikeblas (talk) 19:56, 12 July 2020 (UTC)
"I'm not even going to consider..."
That's OK, you're not required to participate. But in declaring that you're not participating, please don't selectively quite only parts of a guideline, as you do - unnecessarily garishly - above. The guidleline also says, near the top of the page (and so before the subsection you quote):"There are three related measures of an article's size: Readable-prose size [...] Wiki markup size [and] Browser-page size"
. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 12:05, 2 January 2020 (UTC)- Yes, and the guideline says absolutely nothing about hard-limits on markup size like it does with the prose size, which you clearly are trying to use as a justification for splitting pages based upon their on markup size. The thing is, this page might have a markup size in the 200+kB range, but both this script and xtools indicate that the prose size is 49-50kB. No one is going to buy what you are selling if you keep trying to argue that this page needs to be split based upon markup size. And even if that was an issue, which it clearly is not, I could simply move this entire page's source code to a template and transclude it in, causing this page's markup size to drop to <10kB while the readable prose size remains the same. Seppi333 (Insert 2¢) 12:14, 2 January 2020 (UTC)
- Not so; for example the guideline says:
"The text on a 32 kB page takes about five seconds to load for editing on a dial-up connection, with accompanying images taking additional time, so pages significantly larger than this are difficult for older browsers to display. Some large articles exist for topics that require depth and detail, but typically articles of such size are split into two or more smaller articles. "
. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 12:21, 2 January 2020 (UTC)- I don't see a hard/fixed limit anywhere in the text you quoted. It appears to assert the incredibly obvious fact that shittier connections load webpages more slowly, and we're talking about two-tenths of one megabyte. Seppi333 (Insert 2¢) 12:26, 2 January 2020 (UTC)
- Wikipedia talk:Article size is available, should you wish to dispute the contents of the guideline. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 12:50, 2 January 2020 (UTC)
- @Pigsonthewing: Dispute the guideline? Why would I do that? It supports my assertion and contradicts yours. I'm actually happy with it the way it is. Also, thank you again for the nomination. You're doing me a favor here. Seppi333 (Insert 2¢) 13:15, 2 January 2020 (UTC)
- Wikipedia talk:Article size is available, should you wish to dispute the contents of the guideline. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 12:50, 2 January 2020 (UTC)
- I don't see a hard/fixed limit anywhere in the text you quoted. It appears to assert the incredibly obvious fact that shittier connections load webpages more slowly, and we're talking about two-tenths of one megabyte. Seppi333 (Insert 2¢) 12:26, 2 January 2020 (UTC)
- Not so; for example the guideline says:
- Yes, and the guideline says absolutely nothing about hard-limits on markup size like it does with the prose size, which you clearly are trying to use as a justification for splitting pages based upon their on markup size. The thing is, this page might have a markup size in the 200+kB range, but both this script and xtools indicate that the prose size is 49-50kB. No one is going to buy what you are selling if you keep trying to argue that this page needs to be split based upon markup size. And even if that was an issue, which it clearly is not, I could simply move this entire page's source code to a template and transclude it in, causing this page's markup size to drop to <10kB while the readable prose size remains the same. Seppi333 (Insert 2¢) 12:14, 2 January 2020 (UTC)