User talk:Headbomb/Archives/2008/July
| This page is an archive of past discussions. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
Thanks for your !vote at my RFA

Thank you, Headbomb, for your support !vote at my RFA. I will be doing my best to make sure that your confidence has not been misplaced. --lifebaka (Talk - Contribs) 18:46, 1 July 2008 (UTC)
Convenience
Linking to it also is more convenience. As it stands now transcluded, people have to click on the "edit" button and then copy and paste the link to the sandbox into their address bar. Nothing is gained from transcluding it and it only makes people do an extra step of work. hbdragon88 (talk) 02:24, 6 July 2008 (UTC)
What censor died and made you boss?
If you want to advance a counter argument to what I wrote, be my guest. But don’t try that horse shit again of deleting what I posted. If you want to be helpful, you are free to edit my last post with a better link to a more suitable vote. Greg L (talk) 01:04, 7 July 2008 (UTC)
Please remain WP:Civil. If you have steam to blow, go take a walk or watch TV for a while. Making remarks like that when your "hot" just leads to a heap of trouble. Headbomb {ταλκ – WP Physics: PotW} 01:06, 7 July 2008 (UTC)
- I’m just not seeing incivility in that post: just plain ol’ fashioned plain-speak. I don’t choose, this evening (and with that editor) to hold my tongue. In fact, I would be interested in hearing from you, to cite the single most uncivil sentence from that post that goes beyond any reasonable civilized debate. I don’t think I used any technique that you can’t find in college-level debate class. If some noob wants to cite non-sense rules (“it’s uncivil to ignore me”), I’ll call it for what it is. You can go ahead and play the good cop here. I’m in a mood right now to do a little crap cutting. Greg L (talk) 01:15, 7 July 2008 (UTC)
- Alright then let's cut the crap. I don't like the tactics of the opposition anymore than you do, I don't like how they don't seem to get the WP:Point, I don't like how they accuse me of editing out of bad faith, or that I've somehow biased the process, or that consensus is invalid because there was 11 people who voted against the deprecation 5-6 months ago, etc... However, no matter how much I may not like these tactics and the general way they debate things, it's not by posting comments such as "Do all protons have to decay before you start making sense?" (paraphrased) that are purposefully inflammatory in nature. Do you think the last "vote" we had would carry any kind of legitimacy if I replied to Thunderbird with comments such as "Well until you decide to make sense, which is unlikely to happen before the sun burns out, I'm going to ignore you?" [I’d pay five bucks to your PayPal account if you did. :-) Greg L (talk) 02:04, 7 July 2008 (UTC)] Not gonna happen Headbomb {ταλκ – WP Physics: PotW} 02:09, 7 July 2008 (UTC)instead of comments such as "Please substantiate your arguments. If you are not willing to do so, then this discussion is over."?
- The "meat" of both comments are the same, but the former is inflammatory will the later is not. Headbomb {ταλκ – WP Physics: PotW} 01:40, 7 July 2008 (UTC)
- There are “personal attacks”, which Wikipedia defines as “threatening legal action”, “racist comments”, “death threats”, etc. Then there is being “uncivil”, which is more nebulous, but sorta vacuums up stuff like, “you’re an ass who should be ignored”, or “you’re stupid and don’t know what you’re talking about.” Extensive language like that is prohibited; particularly when it impedes progress. What I did was A) plain-speak, that B) was (perhaps) “inflamatory”. So what? That’s my choice. Get this one straight: the proper response to bad speech is better speech—not censorship. Don’t revert me again please; particularly when your only complaint doesn’t amount to anything more than it’s ‘inflammatory and might set our cause back.’ My thoughts. My speech. Greg L (talk) 02:00, 7 July 2008 (UTC)
Your RfA
I'm afraid I withdrew your RfA per WP:SNOW. Concerns focused mainly on the short time between your two RfAs. Best, PeterSymonds (talk) 22:22, 8 July 2008 (UTC)
- Expect to be bothered for template-editing in the next few months, why is why I applied in the first place. If you re-opened and vouched for me, perhaps that would make people reconsider, but I can't tell you what your opinion is.Headbomb {ταλκ – WP Physics: PotW} 22:33, 8 July 2008 (UTC)
- Sorry, man, but it was kinda' gonna' happen. I wish you'd asked some people for advice before doing that, since they could've said just how negatively the regulars at RFA look at repeat noms with such little time between them. I just want you to know that no one thinks you're not a great editor, and whatever reasons some have for opposing your RFAs they are not trying to say anything about your contributions themselves. Having RFAs that go poorly is not an indication that people don't value you. That said, I can completely understand if you are not feeling very well about the whole 'pedia right now and want to take a break. Really, I suggest you take a few hours off, at least. Go watch a movie; Hancock was pretty good, and I hear Wanted isn't bad as long as you accept that physics doesn't apply to them. Drop me a line if you need anything done, like more {{editprotected}} on templates. Cheers, man. --lifebaka (Talk - Contribs) 22:32, 8 July 2008 (UTC)
- As you're online, I've unprotected {{Physics}} for 24 hours. Pleeease try and keep the edits down to a minimum! :) The job queue is currently at 2 million or something ridiculous. By the way, there was nothing I could have said at that RfA which would change the way things were going. I'm sorry for that, but the outcome would have been no different. All the best, PeterSymonds (talk) 22:40, 8 July 2008 (UTC)
- Heh, never mind, it's already unprotected by lifebaka. Best, PeterSymonds (talk) 22:41, 8 July 2008 (UTC)
Your RfA and "needing" the tools
Hi,
I've just read through your two RFAs.
I'm afraid that it was pretty inevitable that they would fail. In general, people are looking for potential admins who are looking to assist in areas where they are uninvolved, rather than areas where they are involved, and have a potential COI.
The admin tools aren't there to allow you to be a power editor with greater access levels on your own subject of interest (indeed, using admin status to make edits to protected templates in this way is pretty close to abuse of the tools).
Running again after only a week was not a good idea. Sure you don't think the opposers were right, but we allow people an opinion, even if their opinions aren't good ones in our view. Running again, without taking the time to address the concerns (however invalid) appears to be showing an arrogant contempt for other editors (whether that was your intent or not).
Doing this has also seriously impaired your chances in future. Normally, you are expected to wait 2-3 months before trying again. Having tried a second time after only 1 week, you have pretty much ensured that any further RFA in the next 6 months will fail.
Certainly having to wait for an admin to edit a protected template is a PITA, but protected templates that would damage a lot of pages if wrecked are like that for a reason. Having to have two editors involved in getting them changed is a good idea (IMHO), and 24 hours delay isn't a major problem.
Why not work up the changes that you want to make in your user space, test them, then make your admin request, pointing to the revised version in your user space (saves so much explanation)
Mayalld (talk) 06:38, 9 July 2008 (UTC)
- And it's a damn shame that people can't judge things in context and would rather assume bad faith. I took over WP:PHYS about 4-5 months ago, and there's not been a single complaint about my job, and not one single user who accused me of "ownership". I've given absolutely no indication that I would abuse the tools, at best people have shoddy correlations. I ask for feedback, I've not made one disruptive edit here, I haven't been blocked, slapped with an RfC, slapped with 3RR warnings, I correct my mistakes swiftly when pointed out etc... The "tools" may not be designed for power-editing, but their use is what you make of. A chair isn't "designed" to be a weapon, but it can be wielded as one. Coolers may not be designed to be a chair, but that certainly doesn't stop campers from around the world to sit on them. WP:IAR exists for exactly this reasons - when "rules" are in the way of productivity, ignore them. WP:BUREAUCRACY exists for when process gets in the way of productivity. WP:BOLD is there because you need people who show outside the box thinking skills, and who are willing to do things in a new way, or use things in ways they haven't been used before.
- As for taking the time to address these concerns, I've added 6 extra questions exactly on these concerns, with one specifically on self-introspection, and greatly expanded my answers to the three previous ones to clarify what I wanted to do with the tools, and that I didn't plan to be involved in WP:AIV and the like , encouraged people to ask as many questions as they wanted (cause for some weird reason people accuse either of weird things when they ask questions to candidates on the RfA), whether they had 10 or 100.
- This is not a matter of one not addressing concerns, it's a matter of people not wanting their concerns to be addressed. And that's what's wrong with the RfA. Headbomb {ταλκ – WP Physics: PotW} 11:44, 9 July 2008 (UTC)
- As for template-editing, I do use my sandbox to test things (see my sandbox' history]). However, some templates depends on templates, which depends on templates. To recreate that whole tree-structure in users pages is incredibly tedious, especially if all you have to fix in the 3rd level-template is a typo, or add an extra input, and you become more a lot more prone to error. Headbomb {ταλκ – WP Physics: PotW} 11:44, 9 July 2008 (UTC)
Thunderbird2
I don't like how Thunderbird2 keeps on spamming talk pages with his version of events (regarding consensus and deprecation) especially when it is obvious that the talk archive completely disproves his version as misrepresentation. So, what can we do about it? Fnagaton 18:07, 8 July 2008 (UTC)
- I'm afraid than this is similar to the Isrealo-Palestinian conflicts. There's just no-end to it because the proponents are to busy hating each other than trying to find a solution. Whenever a voice of reasons show up, someone feels ignored and blows himself up or decides to "counterstrike". This is a typical case of people not wanting to back down.
- It's a case of someone wanting something, but not willing to argue for it. His rants are all form and no meat. I you figure something out, lemme know, cause there's a bunch of creationists on another forum I would like to shut up once and for all. Headbomb {ταλκ – WP Physics: PotW} 18:18, 8 July 2008 (UTC)
Template for BDprefix
Good idea. :) Fnagaton 09:57, 10 July 2008 (UTC)
'twas Potatoswatter's. Headbomb {ταλκ – WP Physics: PotW} 11:21, 10 July 2008 (UTC)
RfA
Is the worst process on Wikipedia. Go write articles. You'll enjoy it more. —Giggy 07:28, 9 July 2008 (UTC)
- Wholehearted agreement from me here. It really should inspire itself from the FAC and FLC process. Headbomb {ταλκ – WP Physics: PotW} 11:48, 9 July 2008 (UTC)
- Heh. But why not GA? ;-) —Giggy 11:57, 9 July 2008 (UTC)
- Perhaps, but I never went there (well I dropped by, but only to look up for new physics GA candidates to update Wikipedia:WikiProject Physics/Current activity) and I'm not really familiar with its process and criteria. It's probably very similar to FLC and FAC, but I'm not the kind of person who recommends things they aren't familiar with or who would pass judgment on things without having the full picture. Headbomb {ταλκ – WP Physics: PotW} 12:03, 9 July 2008 (UTC)
- Will never be like FAC or FLC because most people, including me, believe that it takes more than a week to change a person's behavior ;) With that being said, I think that the informal "3 month minimum" between RFAs is perhaps too strict; it should vary depending on how often a person is on Wikipedia, methinks. Gary King (talk) 08:22, 11 July 2008 (UTC)
- Perhaps, but I never went there (well I dropped by, but only to look up for new physics GA candidates to update Wikipedia:WikiProject Physics/Current activity) and I'm not really familiar with its process and criteria. It's probably very similar to FLC and FAC, but I'm not the kind of person who recommends things they aren't familiar with or who would pass judgment on things without having the full picture. Headbomb {ταλκ – WP Physics: PotW} 12:03, 9 July 2008 (UTC)
- Heh. But why not GA? ;-) —Giggy 11:57, 9 July 2008 (UTC)
- We'll have to agree to disagree then. If life taught me one thing its that whenever you fall back on hard limits to make judgments, instead of taking the entirety of the situation into account, your going to make bad judgments (see reply to Giggy for a similar statement). Limits should be rules of thumb, not rules. Do most people change in a week's time? No, you'll find agreement from me here. But that doesn't mean that it's impossible.
- The bunch of bad reasons in my first RfA made me lose perspective (WP:UAA, Kurt's self-nom=autofail, a bad sig I used for 2 days or so, accusations of canvassing...), I admit that much. People wanted more edit summary usage, and it's a sensible requests than admins should document their edits, so I've turned on the reminder feature and I use them all the time now. It doesn't take me three months to realize that I've lost perspective in my first RfA, nor to rectify and improve my behaviour. Headbomb {ταλκ – WP Physics: PotW} 12:29, 11 July 2008 (UTC)
- Sure falling back on hard limits makes for bad judgements sometimes. However, you need to bear in mind that the purpose of RfA is to;
- ensure that WP has enough people with the right skills and attitude given admin status
- ensure that few if any people without these skills and attitudes are given admin status
- By these two criteria, the process is working fine. Mayalld (talk) 13:29, 11 July 2008 (UTC)
- Sure falling back on hard limits makes for bad judgements sometimes. However, you need to bear in mind that the purpose of RfA is to;
- I wasn't involved in your RFA, so I'm not too aware of what happened there. Just FYI, I had an RFA that failed, too. The opposes I received were actionable, so I appreciate that and act on them. Perhaps some people can act on all their opposes in a week, but I don't think it's in RFA's best interest to have an RFA fail, the editor act on their opposes, then return to RFA a week later. You really have to look at it from another person's perspective – it doesn't look good. Gary King (talk) 17:56, 11 July 2008 (UTC)
- Yeah well I'm an idealist.. Headbomb {ταλκ – WP Physics: PotW} 17:57, 11 July 2008 (UTC)
Congratulations on getting what appears to be your first successful WP:FL during the last month. You may want to get involved in our List of the Day/List of the Month experiment. Feel free to help us select next months lists at User:TonyTheTiger/List of the Day/voting/200808 or nominate your list for consideration to be a LOTD in September at User:TonyTheTiger/List of the Day/Nominees/200809.--TonyTheTiger (t/c/bio/WP:CHICAGO/WP:LOTM) 21:02, 11 July 2008 (UTC)
Template:Physics
I have reduced the editing protection on Template:Physics so that you can freely make changes. Please let me know—on my talk page—once you're done so that I may re-protect the page. Thanks, {{Nihiltres|talk|log}} 15:26, 12 July 2008 (UTC)
Signpost links
Fixed; thank you. Tony (talk) 16:21, 12 July 2008 (UTC)
Thanks for the feedback. Still, plese check the reply. Thanks! Nergaal (talk) 20:18, 12 July 2008 (UTC)
Articles you might like to edit, from SuggestBot
SuggestBot predicts that you will enjoy editing some of these articles. Have fun!
SuggestBot picks articles in a number of ways based on other articles you've edited, including straight text similarity, following wikilinks, and matching your editing patterns against those of other Wikipedians. It tries to recommend only articles that other Wikipedians have marked as needing work. Your contributions make Wikipedia better -- thanks for helping.
If you have feedback on how to make SuggestBot better, please tell me on SuggestBot's talk page. Thanks from ForteTuba, SuggestBot's caretaker.
P.S. You received these suggestions because your name was listed on the SuggestBot request page. If this was in error, sorry about the confusion. -- SuggestBot (talk) 22:23, 12 July 2008 (UTC)
Misrepresentations from T-bird
Please see User talk:Rlevse#Request for advice (part II). T-bird sought the “advise” of a super administrator over my swatting him down by claiming “The net result is to discourage meaningful discussion, giving a false impression of consensus.” Greg L (talk) 00:56, 13 July 2008 (UTC)
Request for mediation at MOSNUM
I have completed a request for cabal mediation here. Thunderbird2 (talk) 15:31, 13 July 2008 (UTC)
You say aligning the table in this article causes a problem. What is the nature of the problem? Spacepotato (talk) 06:21, 15 July 2008 (UTC)
Well basically, the word wrap doesn't work like it should, and the table overlaps some text, and is stuck halfway between two headers. I'll take a screenshot. Headbomb {ταλκ – WP Physics: PotW} 06:33, 15 July 2008 (UTC)
Screenshot. Headbomb {ταλκ – WP Physics: PotW} 06:37, 15 July 2008 (UTC)
- There's no overlap there. Actually, it looks fine, although it would be more aesthetically pleasing if the table had a blank border surrounding the box. Spacepotato (talk) 06:43, 15 July 2008 (UTC)
- Look carefuly, you'll see the text goes over the table. Even if it didn't go over the table, there needs to be some spacing between the text and the table. Also the table goes right over the DAV Start header. I don't know what exactly is the problem, (MediaWiki software?), but it looks like the text does what it's supposed to be, and that it's the table that is shifted up+left. Headbomb {ταλκ – WP Physics: PotW} 06:47, 15 July 2008 (UTC)
Please do not vandalize my userpage...
...in ways that are ugly. Seriously, five <big>s is not hot. Next time, I suggest a healthy dose of ![]() . Cheers! --lifebaka (Talk - Contribs) 16:03, 15 July 2008 (UTC)
. Cheers! --lifebaka (Talk - Contribs) 16:03, 15 July 2008 (UTC)
- Well, vandalism usually is made to uglify things, so ... :P. Headbomb {ταλκ – WP Physics: PotW}
Your AWB edit to Uncertainty
Your AWB edit to Uncertainty doesn't make sense to me. The sort order seems to have been disrupted by the edit. What is the explanation? --AB (talk) 05:35, 20 July 2008 (UTC)
I think the explanation is because the alphabetical ordering of abbreviated versions of a language (in the sidebar) is not the same as the full version ordering. Headbomb {ταλκ – WP Physics: PotW} 12:45, 20 July 2008 (UTC)
- I checked and that is indeed the explanation. Headbomb {ταλκ – WP Physics: PotW} 12:58, 20 July 2008 (UTC)
- What is meant by full version ordering here? I can't make out any sort order here. From what I understand, the entries should be in alphabetical sort order, and they're not. --AB (talk) 17:36, 20 July 2008 (UTC)
Check the language bar on the left. What I mean by full version is the non-abbreviated version. For example, fr stands for French, it stands for Italian etc... Well some language can be abbreviated in a way that does not preserve the alphabetical order. Suppose Cantonese (chinese) is abbreviated into ma, and that German is abbreviated into ge. Cantonese comes before German alphabetically speaking, but to preserve that, you need to place ma before ge. Headbomb {ταλκ – WP Physics: PotW} 19:15, 20 July 2008 (UTC)
- I had hoped that the languages in the language bar were sorted automatically, irrespective of their ordering in the typed list in the article's wiki text, but it seems that this feature has not been implemented. --AB (talk) 19:58, 20 July 2008 (UTC)
Thousand million or million million?
You removed a link in General relativity to billion. This link is needed because some people believe that "billion" means "million million" which we call "trillion". I reverted you. JRSpriggs (talk) 09:09, 19 July 2008 (UTC)
- I think the link is unnecessary. Anyone who still thinks a billion means that needs a time-warp back to the 1940s. (Except that in India there's a state of confusion about it.) Tony (talk) 06:48, 27 July 2008 (UTC)
your AWB edits ..
hello. i've notice that your awb edits of late have been adding spaces between the {{lifetime}} template and the category stacks upon which it sits. while seemingly benign, it is actually causing more problems than it is intended to repair. it would be great if you could work that bug out before continuing with those minor fixes. --emerson7 00:17, 21 July 2008 (UTC)
Ah. Well I don't know what causes the bug, so you might want to file a bug report. In the meantime, I'll stop placing spaces between the lifetime templates. Could you give me a link to an article where it caused a problem? Headbomb {ταλκ – WP Physics: PotW} 01:26, 21 July 2008 (UTC)
Thx for reverting!
User_talk:Caerwine#You_dot_es_dot. Note the tag on Caerwine's user page concerning rotary telephone dials! Tony (talk) 06:46, 27 July 2008 (UTC)
- PS Please inform me if you need back-up on reverting the other damage I see in his/her contributions list. Tony (talk) 06:56, 27 July 2008 (UTC)
Wikiproject Physics
Thank you for notifying me of your attempts to revitalize this project. My reason for participating earlier was that I am a retired academic who researches in computational and quantum chemistry, but who also on many occasions taught the whole of physical chemistry. I am concerned that articles in this overlap area between chemistry and physics do not get the balance right between the needs of readers from the two disciplines. For example, I think the vast majority of articles on thermodynamics are quite useless to chemists. They have been dominated by physicists who concentrate on rigor and mathematical derivations, while chemists generally do not have the background to grasp that easily. I have tried to work on this with Entropy but I get nowhere. I started the Wikipedia:WikiProject Physical Chemistry with the hope that might help discussion between chemists and physicists, but it is largely inactive. I have other interests on wikipedia and in wikimedia in general and I seem to have less time anyway for working here. Nevertheless I would hope we could try to work through these issues. --Bduke (talk) 22:26, 25 June 2008 (UTC)
Well I would find it hard to remove the mathematical rigor from articles such as entropy, Gibbs free energy, Enthalpy, Joule-Thomson effect, etc, as accuracy is extremely important but there certainly is a place in there for a chemistry-oriented treatment and explanations for the layperson. I believe that it is possible to explain any topics within the real of naturals sciences in a simple and accurate way. If at any point in an article you ask "why" and the explanation is not offered in the next line, then the article is not as good or not as well-structured as it should be.
The best way to solve this problem is to ask "Why is this? on the talk page for everything that is not 100% clear or intuitive. Perhaps Count Iblis and Likebox could offer additional insight. Headbomb {ταλκ – WP Physics: PotW} 23:50, 25 June 2008 (UTC)
- Well, I am not of course asking for mathematical rigour to be removed. What I think we should have, and I think it is a WP guideline somewhere, is a first leading paragraph that says why the topic is important and what in general terms it is all about. At times, the entropy article lead has got close to that, but it always sinks back to obscurity as people change it to make sure there are no errors there. There is a difference between the way that chemists think and are taught compared with physicists. Chemists start by a simplification (that physicists say is simplified to the point of error) and then work towards a better and more accurate understanding with time. Physicists want to start with absolute rigour. We just think differently. I do not know what the solution is.
- The other problem is that of mathematical background. I think I might be coming from the extreme worst case. I have just been involved in a survey of research and teaching of quantum molecular science (quantum chemistry). The responses were quite revealing. Most chemistry departments here in Australia reported that levels of mathematical knowledge of students had been falling rapidly and financial pressures were forcing the discontinuation of courses in quantum chemistry as they were perceived to be difficult. My part of this was the Australian survey, but similar surveys have been carried out in other countries and I have seen some of them. None report this discontinuation of courses and falling off of mathematical knowledge, although the responses I have seen do not include the UK and the USA, where I think it might be a problem. Chemists these days just run from equations. Yet they need to learn about things like entropy. I suspect more people read that article because they came across it in a chemistry course than those who came across it in a physics course. Physics numbers are falling. Straight chemistry numbers are falling, but those in courses on medicinal chemistry, biological chemistry etc are rising and they have poorer mathematics yet still need to learn some thermodynamics.
- We need to recognize our audience more and recognize that there is a problem. Then we might work towards solutions. --Bduke (talk) 00:53, 26 June 2008 (UTC)
I do not think that physics numbers are falling, although I agree that physics curriculum are lighter than they used to be. I think the core of the problem comes from the historical difference between chemistry and physics. The two used to be considered different sciences, but since the arrival of quantum mechanics, it's become clear that the distinction from chemistry and physics is fundamentally non-existent. "Fundamental" chemistry is completely indistinguishable from atomic and molecular physics. The study of chemical reactions is completely indistinguishable from statistical mechanics.
Rutherford had it right when he said that all science was physics or stamp collecting. I don't know if this is sad or not, but I know that biochemistry is the next thing that'll be absorbed into physics, as fundamental biochemistry is nothing more than a branch of chemistry... which is nothing more than a branch of physics. Either way, I'm of the opinion that if one can't do it from the bottom up, there's something missing in one's understanding.
As a small factoid, I'm a former student of chemistry, and I switched to physics after a year because it pissed me to no end that they worked with empirical laws that came out of the blue, and that were never derived from core principles. Also I was bothered by the fact that to no one seemed to define things very precisely, and there were often "cutoffs" that were completely arbitary. Things like the distinction between a covalent bond and a ionic liaison was whether or not the difference in the electronegativity of the two atoms was greater or lower than 1.7 and what happened at a difference of 1.69 or 1.71 was dismissed with hand-waving. Perhaps this was due to bad teachers, or perhaps it was because it was the intro-classes, but I can't help but feeling that there's a general attitude in chemistry that it's OK to not explain things from the bottom up (top down approaches can be useful to introduce things, but always end up doing more harm than good in the long run if that the only thing one has been exposed too). Headbomb {ταλκ – WP Physics: PotW} 01:32, 26 June 2008 (UTC)
- Some deep issues here. I used to take the full reductionist program that everything is reducible to physics, but I am beginning to doubt it. Far from this view becoming more established it is becoming more challenged. The newish Philosophy of chemistry asserts that chemistry is autonomous and not reducible to physics. Can chemical structure be derivable from quantum mechanics? Only with a bit of a fudge, they say.
- "The study of chemical reactions is completely indistinguishable from statistical mechanics." is completely wrong for the majority of chemists who study chemical reactions - i.e the majority who do not study small molecule or atom reactions in molecular beams or other low pressure gas phase experiments, but in the mess of solution. They rarely think about statistical mechanics.
- You have described the teaching of chemistry very well. However, we do not agree that this fuzziness does more harm than good. It trains an amazing instinct that good experimental organic and inorganic chemists have. Chemistry teaching is not changing to be more bottom up, and I do not think it is going to. There IS a fundamental difference between the way chemists and physicists see the teaching of their respective subjects. In the old days, like when I was a student in the late 1950s, it did not matter, as all chemistry students had a good grounding in physics and one could pretend that quantum chemistry courses should be approached like physics. The students played with both ways of learning. In the last two decades of my teaching I hardly ever had a student who had done physics in 1st year or even in the last year at school. They no longer know how to think as a physicist, only as a chemist. Of course there are exceptions and, as I suggest above, Australia may be different from many other countries, but Britain was going the same way when I last taught there in the early 1980s. China, Japan and Europe mostly still have the old way, but many other countries do not. --Bduke (talk) 02:25, 26 June 2008 (UTC)
Indeed the issues are complex. However, the non-reductionist viewpoint of philosophy of chemistry (or philosophy of biology) strikes me as a completely non-scientific attempt to regain the special status biology and chemistry ones had within science, that physics is not "all there is to it". Religion was successfully purged from science, let's not re-introduced it. We have enough trouble fighting the pseudo-scientific nonsense out there as it is.
I'll clarify what I mean by "The study of chemical reactions is completely indistinguishable from statistical mechanics". I mean that at the fundamental level, there is nothing indistinguishable between the two. The distinction bewtween chemical kinetics and statistical mechanics exists only in the same sense that there is a distinction between "order" and "class" in biology.
As for the harmfulness of the "fuzziness", chemists are experiencing it right now when they get confused by rigorous treatments of "chemistry topics". They do not have the mathematical background to truly understand what they are studying. Currently there is only one chemistry student with a physics background that goes higher than 1rst year physics (he took a modern physics class, and statistical thermodynamics), and he is the only one correcting the teacher when he confuses "proportional to" and "equal to", uses equations that are not even unit-consistent, or tries to shove a non-rigorous definition concerning core concepts of chemistry such as the chemical potential etc... It's one thing to not see the rigorous derivation of Gibbs free energy, but it's quite another to be told that the Gibbs energy is defined as being "the useful energy" in a reaction. Headbomb {ταλκ – WP Physics: PotW} 19:08, 30 June 2008 (UTC)
- Oh dear, it is difficult to know where to begin. Let me start with something simple. By "fuzziness", I do not mean getting units wrong or confusing "proportional to" and "equal to" and so one. That misses the point.
- The key point is your view of reductionism and the philosophy of chemistry. As I said earlier, I am mostly a reductionist. I am after all a quantum chemist and I have published some papers in physics journals, such a "Journal of Chemical Physics" and "Molecular Physics". My wife, who years ago did half a BA and a whole MA in Philosophy thinks I am arrogant. O boy, would she go to town on you. Have you read any of the books and papers on philosophy of chemistry? Some are referenced in that WP article. I find it very hard indeed to think that Philosophy of Chemistry, J. van Brakel, Leuven University Press, 2000, is pushing religion or pseudoscience or even trying to regain special status. It is hard going for me, as I am not a philosopher, but it clearly a proper scholarly attempt. I suggest you look at it. I also suggest a look at the journals listed as the first two external references. The standard of articles varies, but there is some hard stuff.
- Now for a hard question. How does molecular structure come from quantum mechanics rigorously? Quantum mechanics tells us that CO2, with both O atoms oxygen-16, consists of 22 indistinguishable electrons, one carbon atom and two indistinguishable oxygen-16 atoms. The chemists structure is 22 indistinguishable electrons in the field of one carbon atom and two distinguishable oxygen-16 atoms. How do we get from one to the other? Molecular structure clearly has distinguishable atoms. It does not matter for molecular structure whether the two O atoms are both O-16 or one O-16 and one O-18, but it does for quantum mechanics. I could point you to some papers that discuss this in depth, but I will leave you think about it. --Bduke (talk) 00:24, 1 July 2008 (UTC)
Well I can't say I've reviewed these particular references, but I did read a lot on philosophy, reductionism, emergence, philosophy of science, epistemology, etc... I too would give your wife a hard time and I'd probably accuse her of letting herself be paralyzed by what I call "epistemological fear" (the fear that since you cannot prove 100% that you are right, or cannot disprove that the alternative is wrong, you must assume that both possibilities are equally likely) :P. I can't speak on Brakel particularly, but every attempt to "de-reductionized" science/universe I've read so far has been guilty of a kind of a sort of "Well, I don't like reductionism, as I think it's arrogant/preposterous/removes the mystery in the universe/etc, therefore reductionism is wrong." I might end reading one of these references if they are availible online, but at this point it feels like I've read everything there was to say about reductionism.
As for your question, I can't tell you how molecular structure comes from quantum mechanics exactly, as it involves solving a great deal of very complex equations and that is well beyond my abilities. For the CO2 example, with the approximation that nuclei can be considered point-like, and that self-interaction is negligible, this involves 22 electrons and 3 nuclei = 25 "particles" to consider. This gives an equation to solve that has 24+23+22+21+20+...+1=300 terms!
The way you would build this equation is however simple. First you mark the particles 1,2,3,4...25, (making them "distinguishable for now") total energy of the system is then given by the energy due to electric attraction/repulsion (V), and the kinetic energy of each particle (K). V is something that looks like
(each term is simply the Couloumb interaction)
You can see that the only difference here will be due to differences in charge (aka, the different charges of nuclei and electrons, aka electrons are distinguishable from nuclei, nuclei of different elements are different from the nuclei of other elements).
The kinetic energy term however involves the mass of the particles.
Meaning that particles of different masses are distinguishable, while those of same masses are indistinguishable.
For CO2 there are 3 cases. CO2 made of two O-16, CO2 made of O-16 AND O-18, and CO2 made of O-18. When the oxygen are made of the same isotopes, they are indistinguishable from one another since there is no way to tell which is which (although you could tell appart CO2 made from two O-16 than two O-18. However, when CO2 is made of one O-16 and one O-18, then there is something that is different between the two, and you can distinguish them. It's a simple matter of asking yourself "If I close my eyes and someone flips the molecule around, can I still tell which is which?" If you can tell, then they are distinguishable and needs to be treated as such. If chemistry tells you that two O-16 are distinguishable, then chemistry is wrong.Headbomb {ταλκ – WP Physics: PotW} 01:47, 1 July 2008 (UTC)
- Well, I know all that. It just proves my point that you and I, as typical of a physicist and a chemist, are miles apart. Please read some of the philosophy articles, I drew your attention to, particularly the book by van Brakel. For more from a science, even physics point of view look for papers by B. T. Sutcliffe and R. G. Woolley. One is in a book called "Fundamental World of Quantum Chemistry: A Tribute to the Memory of Per-Olov Lowdin". How structure comes from QM is indeed complex. I spend my time doing what you are talking about, but that is not the issue. I know the two O atoms are not distinguishable, and chemists indeed learn that that means that that every second line in the rotational fine structure of the vibrational spectrum of CO2 is missing, if the atoms are O-16. However, structure is what makes chemistry works and there we think of one atom at say (0.0,0.0, 0.63) and another at (0.0,0.0.,-0.63). Structure allows us to put atoms into place in space. It is a crucial part of chemistry. The subject is not going to stop thinking in this way, just because you tell them they are wrong. The philosophers are not sure that the chemists are "wrong", within chemistry, even if they are "wrong" within physics, like the spectra example I give. I have an open mind on it. It is clear you do not, and that is a problem. --Bduke (talk) 05:01, 1 July 2008 (UTC)
Now that was rather dishonest of you. You tricked me into correcting a statement which you knew was false, then accuse me of close-mindedness on the grounds that I said the statement was false and that I said "If chemistry tells you that two O-16 are distinguishable, then chemistry is wrong". And from there you hint that I'm saying that structure doesn't exist.
I take time to put thought in my responses to a complex issue. I wrote these equations from scratch, it's not a particularly impressive feat, but it does requires some thinking to write them and to gauge what is an appropriate an explanation for the level of technical proficiency involved in the discussion. I don't like being tricked into saying something so one can distort it to their liking, only to accuse me of sayings things I didn't say by taking what I write out of context. If that is how you debate things, then this discussion is over as far as I'm concerned. A shame, since it was an interesting one.Headbomb {ταλκ – WP Physics: PotW} 05:56, 1 July 2008 (UTC)
- I am sorry if you think I tricked you, but I do not think I did. I said "How do we get from one to the other? Molecular structure clearly has distinguishable atoms." and I meant it. You said ""If chemistry tells you that two O-16 are distinguishable, then chemistry is wrong". Now chemistry does indeed say this, in part, because it says structure is important, but chemistry is not wrong, as that notion of structure is very powerful and makes chemistry what it is. Let me try to expand on what the problem is. In attempting to solve the equations of QM for a molecule, we generally apply the Born-Opprenheimer approximation. It is that that changes "22 indistinguishable electrons, one carbon atom and two indistinguishable oxygen-16 atoms" to "22 indistinguishable electrons in the field of one carbon atom and two distinguishable oxygen-16 atoms". We then fix the positions of the nuclei (so they clearly are distinguishable at this level) and calculate the electronic energy which can be more or less (often very less) an accurate solution within that approximation. With accurate gradients of the energy we can move to the minimum energy structure and thus predict structure. Structure comes out of the Born-Opprenheimer approximation. The question is whether this makes it legitimate. I am inclined to say it does, but von Brakel and many other philosophers say it does not. Another example is the question whether the periodic table can be derived rigorously from QM. Eric Scerri argues long and hard that it can not. He has certainly not been convinced by the arguments I have made to him at times.
- Finally, I did not address you first paragraph, where you say that anti reductionism is 'guilty of a kind of a sort of "Well, I don't like reductionism, as I think it's arrogant/preposterous/removes the mystery in the universe/etc, therefore reductionism is wrong".' I agree that is true of many such arguments, but that does not seem to me to be arguments of people like von Brakel. Unfortunately his book is not online and probably not in most university libraries. I think the big philosophy of science argument now is one about whether individual disciplines are autonomous in some sense or whether biology is really a branch of chemistry which is a branch of physics. I am uncertain whether autonomy is true in a rigorous way, but it is sort of true in the practical sense of how say chemists think and operate compared with physicists. It is that last point that is relevant to how we write articles for WP on topics that appear in the curriculum of both physics and chemistry students.
- If we want to continue this discussion, we should, I think, move it to somewhere that more people would see it, or perhaps draw the attention of editors on the Physics Project and the Chemistry Project. What do you think? --Bduke (talk) 06:55, 1 July 2008 (UTC)
In the Born-Oppenheimer approximation, you fix the position of nuclei, you do not fix which oxygen goes at which position.
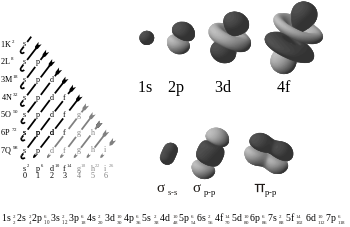
I don't know who Eric Scerri is, but QM completely explains the periodic table.
Solving the hydrogen atom (or any 1-electron atom) yields completely degenerate energy levels (2s and 2p orbitals are completely degenerate). When you introduce another electron (helium for example), then electron-electron repulsion and the s,p,d,f,g,h,i,j,... orbitals modify themselves accordingly. This modification removes the degenerescence, and s orbitals become favored over p orbitals, p over d, d over f, etc... I think a perturbative treatment is usually used, but it could be B-O, I'm not sure. The point is that as you add electrons, the energy degeneracies of the orbitals gets lifted, and the well known ordering of the s,p,d,f,... orbitals emerges. Then as you add other electrons, then spin-spin interactions became important, and that is why you have "exception" in 3d, 4d, 5d, ... (as a note, if you want to treat spin-spin interaction formally, you have to deal with the Dirac equation,not the Schrodinger equation, as spin is due to relativistic effects). The onus is on Scerri to show that QM fails to explain chemistry and that iron is anything more than 26 protons, with a comparable number of neutron, with 26 electrons going around it.
As for the "autonomy" of physics/chemistry/biochemistry/biology science, they will probably always exist in the practical sense in the same way that we say particles physics is different from optics. The only one I'm not sure will end up being within physics' reach is evolutionary and behavioural biology, as these system are just way too massive and complex to approach from a physics point of view.
Feel free to move this discussion elsewhere on wikipedia. Headbomb {ταλκ – WP Physics: PotW} 13:36, 1 July 2008 (UTC)
- [The oxygen atoms] are distinguishable by where they are, and that is fundamental to the concept of molecular structure.
- [Concerning Eric Scerri] well, he has just written a well reviewed book on the periodic table, which I have coming from Amazon. I do not know how much is history and suggestions for new forms, and how much is the philosophical points he has made many times. He is a chemist at Berkeley.
- [As for] the orbitals you are talking about [they] are not a solution to the Schrodinger equation or the Dirac equation. They are a solution, if anything, to the Hartree-Fock equations and that is an approximation which puts a question mark over whether they come from QM. It ignores electron correlation which is vital in getting things right. However that is not the point. Scerri wants QM to predict in one hit the form of the periodic table - length of rows etc. I do not agree with him, but your arguments do not refute his points.
- The consequence of this [autonomy of physics/chem/...] is that chemists, say, do not spend all their time trying to understand everything in terms of physics. They understand things in their own terms and that is a kind of practical autonomy that has real significance. The philosophical autonomy is something else which I agree has not been established, but it is raising some very interesting issues.
-
OK.I'm a bit busy today, but I will raise a discussion on the Physical Chemistry Project, drawing attention in passing to this discussion. I will point people to it from both the Chemistry and Physics Projects. I hope you will encourage this, as I think we really do need a dialog between chemists and physicists about the articles that come under both subjects. Many thanks for this interesting discussion. --Bduke (talk) 22:39, 1 July 2008 (UTC)
First, about the distinguishability of the oxygen atoms. In physics, distinguishable means that if you close your eyes, exchange the two atoms (or particles), and can't tell the difference when you open your eyes, the particles are indistinguishable. I've never met any other definition, and this version of distinguishability is used everywhere in thermodynamics and quantum mechanics, but it is usually first encountered when it comes to deriving the M-B, F-D and B-E distributions. Perhaps this is an example of what you mean by physicists and chemists not speaking the same language, but this definition of distinguishability has direct consequences for chemistry (for example, the above distributions), so I would really be surprised that any other definition is used.
Second, the Hartree-Fock equation is an approximation of the Schroedinger equation. If you want to take spin into consideration, then you have to take the Dirac equation (aka the relativistic Schroedinger equation), because spin is a consequence of special relativity. The Hartree-Fock equation cannot give you any information about spin, as it doesn't contain any information about it. Perhaps there is a relativistic Hartree-Fock equation, but then it would be trivial to point that one does not solve the Dirac-Equation, but rather the relativistic Hartree-Fock equation, as you use the former to find approximate solutions of the latter.
I don't really see why Scerri thinks there's a problem with QM's ability to explain the form of the periodic table. Solving Schroedinger's Hydrogen atom yields a crude periodic table if you are OK with lifting degeneracy manually and slapping spin over it ad hoc. Solving the Dirac Hydrogen atom will yield the same table, but you won't have to slap spin as it will naturally be there. You'll still have to lift degeneracy manually. If you don't want to lift anything manually, then you'll have to get your hands dirty and solve the Dirac equation for an n electron atom. If Scerri doesn't want to do that, then he has no grounds to say that QM fails to explain the periodic table.
As for chemists not seeing things in physical terms, I'm not too hot on the idea. It depends on what exactly you mean by "non-physical terms". It's OK to use approximations and empirical rules, but it's quite another to posit these empirical rules as the fundamental reality. Headbomb {ταλκ – WP Physics: PotW} 00:15, 2 July 2008 (UTC)
- We are just talking at cross purposes. On Scerri, if you are not prepared to read his work, then we will not get anywhere. I am only beginning to put his point of view and since I do not agree with it (although I think it is important), I am probably getting it wrong. Exactly the same applies to distinguishability and structure. If you have not read the people who argue that structure is not rigorously obtained from QM, then again we are getting nowhere. On the periodic table, you say "solve the Dirac equation for an n electron atom" and I presume you mean a separate solution for each and every atom. How does that give the periodic table? The periodic table is about similarities and differences between atoms. What is it about a full rigorous solution of the exact QM equations that tells you there is a similarity between O and S? Where did I talk about "non-physical terms"? I talked about thinking in chemistry terms as opposed to physics terms. --Bduke (talk) 00:41, 2 July 2008 (UTC)
Well it's hard for me to tackle what exactly Scerri's point are since I don't have his book, nor can I afford to spend 100$+ on it. However, I don't think I need to read what their actually opposition is since QM does explain the periodic table. Solving the 1 electron atom yields the basic of the "language" of the periodic table. Out of the solution (this one is exact), you'll get the n, m, ml (or simply l), and ms (or simply s) quantum numbers. The hydrogen atom's lowest energy orbital are any orbital in the n=1, so either 1s1 and 1s2 (but we say 1s1 by convention). For the helium atom, there are more than 1 electron, so the s,p,d,f... degeneracy is lifted. However the "character" of s,p,d,f orbital remains as there is a smooth transition from the 1-electron atom to the 2-electron atom solution as you increase (mathematically) the "strength" of electron-electron interaction, in a completely analogous way to the Stark effect and the Zeeman effect. The same applies for when you go from a 1-electron atom to any n-electron atom. That is why it makes sense to compare an [He]2s2 and a [Ne]3s2 configuration).
Crudely speaking n corresponds to the row, m corresponds to the blocks (s,p,d,f,g,h,...) and ml along with ms tells you where you are (horizontally) in the block. The shape of the periodic shape is more of a human concern than of a scientific concern, as it depends on how we feel like organizing it. What is really important is that we are able to explain this sequence, and QM was successful in doing so. I'm afraid I can't say much more on this, as I've not read Scerri, and that neither of us believes he is right.
And by non-physical terms I didn't mean crazy ideas, I just meant terms not encountered in physics. My bad.Headbomb {ταλκ – WP Physics: PotW} 01:15, 2 July 2008 (UTC)
The Scerri book I was referring to is only US$20 or so, and it should by now be in most libraries. The copy I ordered from amazon has just arrived. It is called "The Periodic Table: Its Story and Significance". It looks as if chapers 7 - 9 cover what I was talking about. I'll have a read of it later and get back to you. --Bduke (talk) 04:57, 2 July 2008 (UTC)
Ah, I thought it was a textbook. If I see it at the library, I'll pick it up, but I doubt that they have it here. Lemme know what Scerri says.Headbomb {ταλκ – WP Physics: PotW} 04:59, 2 July 2008 (UTC)
Gluons
When it comes to the notability of the edit, consider that the source of the controversy comes from Chick tracts, which themselves have a Wikipedia article, Chick tract, which is quite long. Chick tracts have been around for over 40 years, having sold over 700 million copies. Consider this before deleting the section added for notability.
I know what the Chick tracts are. One sentence in one of his tract is not notable. See WP:UNDUE and WP:NOTABILITY. Headbomb {ταλκ – WP Physics: PotW} 13:27, 13 July 2008 (UTC)
Nobel icons
Hi Headbomb! A friend of mine tries to convince me that the Nobel icons, ![]() , shouldn't be placed in the infobox of scientists. What do you suggest on this issue, or may you know some way that we can ask for the opinion of the wider community. Cheers, Gülməmməd Talk 23:59, 19 July 2008 (UTC)
, shouldn't be placed in the infobox of scientists. What do you suggest on this issue, or may you know some way that we can ask for the opinion of the wider community. Cheers, Gülməmməd Talk 23:59, 19 July 2008 (UTC)
I got no strong opinion on the subject really. I like to have them around, but I could also live without. Try asking WikiProject Physics, WikiProject Chemistry and WikiProject Biology for feedback for a wider audience. Headbomb {ταλκ – WP Physics: PotW} 03:59, 20 July 2008 (UTC)





