User:Mr. Ibrahem/Eszopiclone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lunesta, Eszop, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605009 |
| License data |
|
| Routes of administration | By mouth (tablets) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 52–59% |
| Metabolism | Liver oxidation and demethylation (CYP3A4 and CYP2E1-mediated) |
| Elimination half-life | 6 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
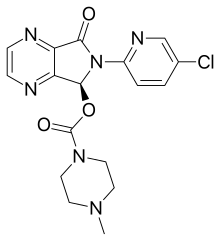
| Formula | C17H17ClN6O3 |
| Molar mass | 388.81 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Eszopiclone, sold under the brand-name Lunesta among others, is a medication used in the treatment of insomnia.[2] Evidence supports slight to moderate benefit up to six months.[3][2][4] It is taken by mouth.[3]
Common side effects include headache, dry mouth, nausea, and dizziness.[3] Severe side effects may include suicidal thoughts, abuse, hallucinations, and angioedema.[3] Greater care is recommended in those with liver problems and older people.[3] Rapid decreasing of the dose may result in withdrawal.[3] Eszopiclone is classified as a nonbenzodiazepine sedative hypnotic and as a cyclopyrrolone.[5] It is the S-stereoisomer of zopiclone.[3] It works by interacting with the GABA receptors.[5]
Approved for medical use in the United States in 2004, eszopiclone is available as generic medication.[3] In the United States, the wholesale cost is about 0.20 USD per dose.[6] In 2017, it was the 214th most commonly prescribed medication in the United States, with more than two million prescriptions.[7][8] Eszopiclone is not sold in the European Union, as in 2009 the EMA ruled that it was too similar to zopiclone to be considered a new patentable product.[9][10]
References[edit]
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 22 January 2021. Retrieved 8 September 2020.
- ^ a b Huedo-Medina, TB; Kirsch, I; Middlemass, J; Klonizakis, M; Siriwardena, AN (Dec 17, 2012). "Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration". BMJ (Clinical Research Ed.). 345: e8343. doi:10.1136/bmj.e8343. PMC 3544552. PMID 23248080.
- ^ a b c d e f g h "Eszopiclone Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 25 October 2010. Retrieved 6 April 2019.
- ^ Rösner, S; Englbrecht, C; Wehrle, R; Hajak, G; Soyka, M (10 October 2018). "Eszopiclone for insomnia". The Cochrane Database of Systematic Reviews. 10: CD010703. doi:10.1002/14651858.CD010703.pub2. PMC 6492503. PMID 30303519.
- ^ a b "Eszopiclone" (PDF). F.A. Davis. 2017. Archived from the original (PDF) on October 8, 2017. Retrieved April 15, 2017.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Eszopiclone - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- ^ "End of Sepracor-GSK Deal Raises Question in Lunesta Patent Fight". www.cbsnews.com. 13 June 2009. Archived from the original on 7 April 2019. Retrieved 7 April 2019.
- ^ "Sepracor Pharmaceuticals Ltd withdraws its marketing authorisation application for Lunivia (eszopiclone)". European Medicines Agency. 15 June 2009. Archived from the original on 1 December 2017. Retrieved 7 April 2019.
