User:Liucatherinek/sandbox
Binding Site[edit]

In biochemistry and pharmacology, a binding site is a region on a protein where ligands bind with high specificity. These sites are geometrically unique to its particular ligand and the binding creates strong and specific binding due to the aggregation of favorable interactions formed at the site with the ligand.[1] The binding event is often accompanied by a conformational change that results in a change of protein function. Bonds formed at these sites are usually transient and non-covalent so that the induced biological activity can be easily regulated. Optimal conditions of a binding site are usually at physiological pH or at the pH of the compartment in which site is located.[2]
Function[edit]
A biomolecule's binding site entails great information about its biological function.[3] For example, the electrostatic charge, steric shape and geometry of the site selectively allow for highly specific ligands to bind, activating a particular cascade of cellular interactions the protein is responsible for.[4][5] Types of ligands include neurotransmitters, toxins, neuropeptides, and steroids.[6] Binding sites incur functional changes in a number of contexts, including enzyme catalysis, molecular pathway signaling, homeostatic regulation, and physiological function.
Catalysis[edit]
Enzymes incur catalysis by binding more strongly to transition states than substrates and products. At the catalytic binding site, a number of different interactions may act upon the substrate. These range from electric catalysis, acid and base catalysis, covalent catalysis, and metal ion catalysis.[7] These interactions decrease the activation energy of a chemical reaction by providing favorable interactions to stabilize the high energy molecule. Enzyme binding allows for closer proximity and exclusion of substances irrelevant to the reaction. Side reactions are also discouraged by this specific binding.[8] [9]
Types of enzymes that can perform these actions include oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases.[10]
For instance, the transferase hexokinase catalyzes the phosphorylation of glucose to make glucose-6-phosphate. Active site residues of hexokinase allow for stabilization of the glucose molecule in the active site and spurs the onset of an alternative pathway of favorable interactions, decreasing the activation energy.[11]
Inhibition[edit]
Protein inhibition by inhibitor binding may induce obstruction in pathway regulation, homeostatic regulation and physiological function.
Competitive inhibitors compete with substrate to bind to free enzymes at active sites and thus impede the production of the enzyme-substrate complex upon binding. For example, carbon monoxide poisoning is incurred by the competitive binding of carbon monoxide as opposed to oxygen in hemoglobin.
Uncompetitive inhibitors, alternatively, bind concurrently with substrate at active sites. Upon binding to an enzyme substrate (ES) complex, an enzyme substrate inhibitor (ESI) complex is formed. Similar to competitive inhibitors, the rate at product formation is decreased also.
Lastly, mixed inhibitors are able to bind to both the free enzyme and the enzyme-substrate complex. However, in contrast to competitive and uncompetitive inhibitors, mixed inhibitors bind to the allosteric site. Allosteric binding induces conformational changes that may increase the protein's affinity for substrate. This phenomenon is called positive modulation. Conversely, allosteric binding that decreases the protein's affinity for substrate is negative modulation.[12][13]
Types of Binding Sites[edit]
Active Site[edit]
At the active site, a substrate binds to an enzyme to induce a chemical reaction.[6][14] Substrates, transition states, and products can bind to the active site, as well as any competitive inhibitors.[6] For example, in the context of protein function, the binding of calcium to troponin in muscle cells can induce a conformational change in troponin. This allows for tropomyosin to expose the actin-myosin binding site to which the myosin head binds to form a cross-bridge and induce a muscle contraction.[15]
In the context of the blood, an example of competitive binding is carbon monoxide which competes with oxygen for the active site on heme. Carbon monoxide's high affinity may outcompete oxygen in the presence of low oxygen concentration. In these circumstances, the binding of carbon monoxide induces a conformation change that discourages heme from binding to oxygen, resulting in carbon monoxide poisoning.

Regulatory (Allosteric) Site[edit]
At the regulatory site, the binding of a ligand may elicit amplified or inhibited protein function.[16][17] The binding of a ligand to an allosteric site of a multimeric enzyme often induces positive cooperativity, that is the binding of one substrate induces a favorable conformation change and increases the enzyme's likelihood to bind to a second substrate.[18] Regulatory site ligands can involve homotropic and heterotropic ligands, in which single or multiple types of molecule affects enzyme activity respectively.[19]
Enzymes that are highly regulated are often essential in metabolic pathways. For example, phosphofructokinase (PFK), which phosphorylates fructose in glycolysis, is largely regulated by ATP. Its regulation in glycolysis is imperative because it is the committing and rate limiting step of the pathway. PFK also controls the amount of glucose designated to form ATP through the catabolic pathway. Therefore, at sufficient levels of ATP, PFK is allosterically inhibited by ATP. This regulation efficiently conserves glucose reserves, which may be needed for other pathways. Citrate, an intermediate of the citric acid cycle, also works as an allosteric regulator of PFK.[19][12]
Binding Curves[edit]
Binding curves describe the binding behavior of ligand to a protein. Curves can be characterized by their shape, sigmoidal or hyperbolic, which reflect whether or not the protein exhibits cooperative or noncooperative binding behavior respectively.[4] Typically, the x-axis describes the concentration of ligand and the y-axis describes the fractional saturation of ligands bound to all available binding sites.[16]

Modeling with binding curves are useful when evaluating the binding affinities of oxygen to hemoglobin and myoglobin in the blood. Hemoglobin, which has four heme groups, exhibits cooperative binding. This means that the binding of oxygen to a heme group on hemoglobin induces a favorable conformation change that allows for increased binding favorability of oxygen for the next heme groups. In these circumstances, the binding curve of hemoglobin will be sigmoidal due to its increased binding favorability for oxygen. Since myoglobin has only one heme group, it exhibits noncooperative binding which is hyperbolic on a binding curve.[20]
Applications[edit]
Biochemical differences between different organisms and humans are useful for drug development. For instance, penicillin kills bacterial enzymes by inhibiting DD-transpeptidase, destroying the development of the bacterial cell wall and inducing cell death. Thus, the study of binding sites is relevant to many fields of research, including cancer mechanisms[21], drug formulation[22], and physiological regulation.[23] The formulation of an inhibitor to mute a protein's function is a common form of pharmaceutical therapy.[24]
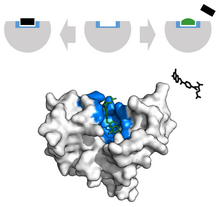
In the scope of cancer, ligands that are edited to have a similar appearance to the natural ligand are used to inhibit tumor growth. For example, Methotrexate, a chemotherapeutic, acts as a competitive inhibitor at the dihydrofolate reductase active site.[25] This interaction inhibits the synthesis of tetrahydrofolate, shutting off production of DNA, RNA and proteins.[26] Inhibition of this function represses neoplastic growth and improves severe psoriasis and adult rheumatoid arthritis.[27]
In cardiovascular illnesses, drugs such as beta blockers are used to treat patients with hypertension. Beta blockers (β-Blockers) are antihypertensive agents that block the binding of the hormones adrenaline and noradrenaline to β1 and β2 receptors in the heart and blood vessels. These receptors normally mediate the sympathetic "fight or flight" response, causing constriction of the blood vessels. [28]
Competitive inhibitors are also largely found commercially. Botulinum toxin, known commercially as Botox, is a neurotoxin causes flaccid paralysis in the muscle due to binding to acetylcholine dependent nerves. This interaction inhibits muscle contractions, giving the appearance of smooth muscle.[29]
Prediction[edit]
A number of computational tools have been developed for the prediction of the location of binding sites on proteins.[30][31][32] These can be broadly classified into sequence based or structure based.[32] Sequence based methods rely on the assumption that the sequences of functionally conserved portions of proteins such as binding site are conserved. Structure based methods require the 3D structure of the protein. These methods in turn can be subdivided into template and pocket based methods.[32] Template based methods search for 3D similarities between the target protein and proteins with known binding sites. The pocket based methods search for concave surfaces or buried pockets in the target protein that possess features such as hydrophobicity and hydrogen bonding capacity that would allow them to bind ligands with high affinity
- ^ Spitzer R, Cleves AE, Varela R, Jain AN (April 2014). "Protein function annotation by local binding site surface similarity". Proteins. 82 (4): 679–94. doi:10.1002/prot.24450. PMC 3949165. PMID 24166661.
- ^ Xu D, Jalal SI, Sledge GW, Meroueh SO (October 2016). "Small-molecule binding sites to explore protein-protein interactions in the cancer proteome". Molecular BioSystems. 12 (10): 3067–87. doi:10.1039/c6mb00231e. PMC 5030169. PMID 27452673.
- ^ Xu D, Jalal SI, Sledge GW, Meroueh SO (October 2016). "Small-molecule binding sites to explore protein-protein interactions in the cancer proteome". Molecular BioSystems. 12 (10): 3067–87. doi:10.1039/c6mb00231e. PMC 5030169. PMID 27452673.
- ^ a b Ahern, Kevin (2015). Biochemistry Free For All. Oregon State University. pp. 110–141.
- ^ Kumar AP, Lukman S (2018-06-06). "Allosteric binding sites in Rab11 for potential drug candidates". PLOS ONE. 13 (6): e0198632. doi:10.1371/journal.pone.0198632. PMC 5991966. PMID 29874286.
- ^ a b c Wilson, K. (2010/03), Wilson, Keith; Walker, John (eds.), "Enzymes", Principles and Techniques of Biochemistry and Molecular Biology, Cambridge University Press, pp. 581–624, doi:10.1017/cbo9780511841477.016, ISBN 9780511841477, retrieved 2018-11-01
{{citation}}: Check date values in:|date=(help) - ^ Wilson, K. (2010/03), Wilson, Keith; Walker, John (eds.), "Enzymes", Principles and Techniques of Biochemistry and Molecular Biology, Cambridge University Press, pp. 581–624, doi:10.1017/cbo9780511841477.016, ISBN 9780511841477, retrieved 2018-11-01
{{citation}}: Check date values in:|date=(help) - ^ J., Dobson, C. M. Gerrard, J. A. Pratt, A. (2008). Foundations of chemical biology. Oxford University Press. ISBN 9780199248995. OCLC 487962823.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Wilson, K. (2010/03), Wilson, Keith; Walker, John (eds.), "Enzymes", Principles and Techniques of Biochemistry and Molecular Biology, Cambridge University Press, pp. 581–624, doi:10.1017/cbo9780511841477.016, ISBN 9780511841477, retrieved 2018-11-01
{{citation}}: Check date values in:|date=(help) - ^ Azzaroni, Omar; Szleifer, Igal (2017-12-04). Polymer and Biopolymer Brushes. doi:10.1002/9781119455042. ISBN 9781119455042.
- ^ Dictionary of Food Science and Technology (2nd ed.). International Food Information Service. 2009. ISBN 978-1-4051-8740-4.
{{cite book}}:|first=missing|last=(help) - ^ a b Brian, Currell R.C. (1997). Biotechnological Innovations in Chemical Synthesis. Elsevier. pp. 125–128. ISBN 978-0-7506-0561-8.
- ^ Clarke, Kim Gail (2013). "Bioprocess engineering". Woodhead Publishing: 79–84. doi:10.1533/9781782421689. ISBN 978-1-78242-167-2.
- ^ Schaschke, Carl (2014). Dictionary of Chemical Engineering. Oxford University Press. ISBN 978-1-62870-844-8.
- ^ Morris, James (2016). Biology How Life Works. United States of America: W.H. Freeman and Company. pp. 787–792. ISBN 978-1-4641-2609-3.
- ^ a b Hardin, Charles C.; Knopp, James A. (2013). Biochemistry - Essential Concepts. Oxford University Press. pp. 51–69. ISBN 978-1-62870-176-0.
- ^ Konc J, Janežič D (April 2014). "Binding site comparison for function prediction and pharmaceutical discovery". Current Opinion in Structural Biology. 25: 34–9. doi:10.1016/j.sbi.2013.11.012. PMID 24878342.
- ^ Fuqua, Clay; White, David (2004), "Prokaryotic Intercellular Signalling", Cell Signalling in Prokaryotes and Lower Metazoa, Springer Netherlands, pp. 27–71, doi:10.1007/978-94-017-0998-9_2, ISBN 9789048164837, retrieved 2018-12-14
- ^ a b Creighton, Thomas E. (2010). The Biophysical Chemistry of Nucleic Acids & Proteins. Helvetian Press. ISBN 978-0956478115. OCLC 760830351.
- ^ Morris, James (2016). Biology How Life Works. United States of America: W.H. Freeman and Company. pp. 835–839. ISBN 978-1-4641-2609-3.
- ^ Spitzer R, Cleves AE, Varela R, Jain AN (April 2014). "Protein function annotation by local binding site surface similarity". Proteins. 82 (4): 679–94. doi:10.1002/prot.24450. PMC 3949165. PMID 24166661.
- ^ Peng J, Li XP (October 2018). "Apolipoprotein A-IV: a potential therapeutic target for atherosclerosis". Prostaglandins & Other Lipid Mediators. 139: 87–92. doi:10.1016/j.prostaglandins.2018.10.004. PMID 30352313. S2CID 53023273.
- ^ McNamara JW, Sadayappan S (October 2018). "Skeletal myosin binding protein-C: An increasingly important regulator of striated muscle physiology". Archives of Biochemistry and Biophysics. 660: 121–128. doi:10.1016/j.abb.2018.10.007. PMC 6289839. PMID 30339776.
- ^ Widemann, B. C. (2006-06-01). "Understanding and Managing Methotrexate Nephrotoxicity". The Oncologist. 11 (6): 694–703. doi:10.1634/theoncologist.11-6-694. ISSN 1083-7159. PMID 16794248.
- ^ Rajagopalan, P. T. R.; Zhang, Z.; McCourt, L.; Dwyer, M.; Benkovic, S. J.; Hammes, G. G. (2002-10-01). "Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics". Proceedings of the National Academy of Sciences. 99 (21): 13481–13486. doi:10.1073/pnas.172501499. ISSN 0027-8424. PMC 129699. PMID 12359872.
- ^ Rajagopalan, P. T. Ravi; Zhang, Zhiquan; McCourt, Lynn; Dwyer, Mary; Benkovic, Stephen J.; Hammes, Gordon G. (2002-10-15). "Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics". Proceedings of the National Academy of Sciences of the United States of America. 99 (21): 13481–13486. doi:10.1073/pnas.172501499. ISSN 0027-8424. PMC 129699. PMID 12359872.
- ^ Food and Drug Administration. "Methotrexate Tablets" (PDF). www.accessdata.fda.gov. Retrieved 2018-12-06.
- ^ Current cardiovascular drugs. Frishman, William H., 1946-, Cheng-Lai, Angela., Newarskas, James. (4th ed.). Philadelphia, Pa.: Current Medicine. 2005. ISBN 1573402214. OCLC 59557150.
{{cite book}}: CS1 maint: others (link) - ^ Montecucco, Cesare; Molgó, Jordi (2005-06-01). "Botulinal neurotoxins: revival of an old killer". Current Opinion in Pharmacology. 5 (3): 274–279. doi:10.1016/j.coph.2004.12.006. ISSN 1471-4892. PMID 15907915.
- ^ Konc J, Janežič D (April 2014). "Binding site comparison for function prediction and pharmaceutical discovery". Current Opinion in Structural Biology. 25: 34–9. doi:10.1016/j.sbi.2013.11.012. PMID 24878342.
- ^ Roche DB, Brackenridge DA, McGuffin LJ (December 2015). "Proteins and Their Interacting Partners: An Introduction to Protein-Ligand Binding Site Prediction Methods". International Journal of Molecular Sciences. 16 (12): 29829–42. doi:10.3390/ijms161226202. PMC 4691145. PMID 26694353.
- ^ a b c Broomhead NK, Soliman ME (March 2017). "Can We Rely on Computational Predictions To Correctly Identify Ligand Binding Sites on Novel Protein Drug Targets? Assessment of Binding Site Prediction Methods and a Protocol for Validation of Predicted Binding Sites". Cell Biochemistry and Biophysics. 75 (1): 15–23. doi:10.1007/s12013-016-0769-y. PMID 27796788. S2CID 6705144.
