User:Chem538w10grp5/sandbox
| This is a Wikipedia user page. This is not an encyclopedia article or the talk page for an encyclopedia article. If you find this page on any site other than Wikipedia, you are viewing a mirror site. Be aware that the page may be outdated and that the user in whose space this page is located may have no personal affiliation with any site other than Wikipedia. The original page is located at https://en.wikipedia.org/wiki/User:Chem538w10grp5/sandbox. |
Ferroelectric Polymers [1] [2] comprise a group of crystalline polar polymers that are also ferroelectric, meaning that they maintain a permanent electric polarization that can be reversed, or switched, in an external electric field.
Ferroelectric polymers, such as polyvinylidene fluoride(PDVF), are used in acoustic transducers and electromechanical actuators because of their inherent piezoelectric response, and as heat sensors because of their inherent pyroelectric response[3].

Background[edit]
First reported in 1971, Ferroelectric Polymers are comprised of polymer chains that must exhibit ferroelectric behavior[4], and possibly, but not required, piezoelectric behavior[3] and pyroelectric behavior[3].
A ferroelectric polymer must contain permanent electrical polarization that can be reversed repeatedly, by an opposing electric field[4]. In the polymer, dipoles can be randomly oriented, but application of an electric field will align the dipoles, leading to ferroelectric behavior. In order for this effect to happen, the material must be below it's Curie Temperature[5]. Above the Curie Temperature, the polymer exhibits paramagnetic behavior, which does not allow for ferroelectric behavior because the electric fields do not align.

A consequence of ferroelectric behavior leads to piezoelectric behavior, where the polymer will generate an electric field when stress is applied, or change shape when an electric field is applied. This is viewed as shrinking, or changes in conformation of the polymer when an electric field is applied, or the by stretching and compressing the polymer, measurable electric fields are generated. Pyroelectric behavior stems from the change in temperature causing electric behavior of the material. While only piezoelectric and ferroelectric behavior are required for a ferroelectric polymer, current ferroelectric polymers also exhibit pyroelectric behavior[3].
In order to have an electric polarization that can be reversed, Ferroelectric Polymers are often crystalline, much like other ferroelectric materials[5]. Ferroelectric properties are derived from electrets, which are defined as a dielectric body that polarizes when an electric field and heat is applied. Ferroelectric polymers differ in that the entire body undergoes polarization, as well as the requirement of heat is not necessary. Although they differ from electrets, they are referred to as electrets often[2]. Ferroelectric polymers fall into a category of ferroelectric materials known as a 'order-disorder'[4] material. This material undergoes a change from randomly oriented dipoles which are paraelectric, to ordered dipoles which become ferroelectric.
After the discovery of PVDF, many other polymers have been sought after that contain ferroelectric, piezoelectric, and pyroelectric properties. Initially different blends and copolymers of PVDF were discovered, such as a polyvinylidene fluoride with poly(methyl methacrylate)[2].
Other structures were discovered to possess ferroelectric properties, such as polytrifluoroethylene[6] and odd-numbered nylon[2][7][8].
History[edit]

The concept of ferroelectricity was first discovered in 1921. This phenomenon began to play a much larger role in electronic applications during the 1950’s after the increased use of BaTiO3. This ferroelectric material is part of the corner-sharing oxygen octahedral structure, but ferroelectrics can also be grouped into three other categories. These categories include organic polymers, ceramic polymer composites, and compounds containing hydrogen-bonded radicals. It wasn’t until 1969 that Kawai first observed the piezoelectric effect in a polymer polyvinylidene fluoride (PVDF). Two years later, the ferroelectric properties of the same polymer were reported. Throughout the 1970’s and 1980’s, these polymers were applied to data storage and retrieval. Subsequently, there has been tremendous growth during the past decade in exploring the materials science, physics, and technology of poly(vinylidenefluoride) and other fluorinated polymers. Copolymer PVDF with trifluoroethylene and odd-numbered nylons were additional polymers that were discovered to be ferroelectric. This propelled a number of developing applications on piezoelectricity and pyroelectricity.
Polyvinylidene Fluoride[edit]
Synthesis of Polyvinylidene Fluoride(PVDF)[edit]
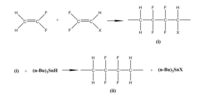
The easiest way of synthesizing PVDF is the radical polymerization of vinylidene fluoride (VF2), however, the polymerization is not completely regiospecific. The asymmetric structure of VF2 leads to the orientation isomers during the polymerization. The configuration of the monomer in the chain can be either “head to head”, “head to tail” or “tail to tail”.

To get more control on the regiospecific polymer synthesis, copolymerization was proposed. One of these methods is introducing the precursor polymer made from copolymerization of VF2 with either 1-chloro-2,2-difluoroethylene (CVF2) or 1-bromo-2,2-difluoroethylene( BVF2). The chlorinated or brominated monomers are attacked at their CF2 carbon by growing –CH2CF2∙ radical. After reductive dechlorination or debromination with tri-n-butyltin hydride they become a reversed VF2 unit in the final polymer.Therefore, a regioisomer of PVDF is formed.[9]
Study of the structure of PVDF[edit]
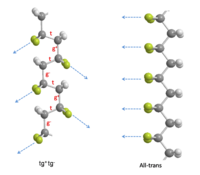
To minimize the potential energy of the chains arising from internal steric and electrostatic interactions, the rotation about single bonds happens in the chain of PVDF. There are two most favorable torsional bond arrangements: trans ( t ) and gauche± ( g± ). In the case of “ t”, the substituents are at 180o to each other .In the case of “g±” , the substituents are at ± 60o to each other. PVDF molecules contain two hydrogen and two fluorine atoms per repeat unit, so they have a choice of multiple conformations. However, rotational barriers are relatively high, the chains can be stabilized into favorable conformations other than that of lowest energy. The three known conformations of PVDF are all-trans, tg+tg-, and tttg+tttg- . The first two conformations are the most common ones and are sketched out in the figure on right. In the tg+tg- conformation, the inclination of dipoles to the chain axis leads to the polar components of both perpendicular(4.0 x 10-30 C-m per repeat) and parallel to the chain(3.4 x 10-30 C-m per repeat). In the all trans structure, the alignment of all its dipoles are in the same direction normal to the chain axis. In this way, it can be expected that the all trans is the most highly polar conformation in PVDF (7.0 x 10-30 C-m per repeat). These polar conformations are the crucial factors that lead to the ferroelectric properties.[3]
Research[edit]
Ferroelectric polymers have propelled many different areas of research based on their properties. These types of polymers have played a role in biomedical and robotic applications and liquid crystalline polymers. In 1974, R.B. Meyer predicted ferroelectricity in chiral smectic liquid crystals by pure symmetry conditions. Shortly after, Clark and Lagerwall had done work on the fast electrooptic effect in a surface-stabilized ferroelectric liquid crystal (SSFLC) structure. This opened up promising possibility of technical applications of ferroelectric liquid crystals in high-information display devices. Through applied research, it was shown that SSFLC structure has faster switching times and bistability behavior in comparison with commonly used nematic liquid crystal displays. In the same time period, the first side-chain liquid crystalline polymers (SCLCP) were synthesized. These comb-like polymers has mesogenic side chains that are covalently bonded (via flexible spacer units) to the polymer backbone. The most important feature of the SCLCP’s is their glassy state. In other words, these polymers have a “frozen” ordered state along one axis when cooled below their glass transition temperature. This is advantageous for research in the area of nonlinear optical and optical data storage devices. The disadvantage is that these SCLCP’s suffered from their slow switching times due to their high rotational viscosity.
Applications[edit]
Nonvatile Memory[edit]
The ferroelectric property exhibits polarization–electric-field-hysteresis loop, which is related to "memory". One application is integrating ferroelectric polymer Langmuir–Blodgett (LB) films with semiconductor technology to produce nonvolatile ferroelectric random-access memory(NV-FRAM or NV-FeRAM) and data-storage devices.Recent research with LB films and more conventional solvent formed films shows that the VDF copolymers(consisting of 70% vinylidene fluoride (VDF) and 30% trifluoroethylene (TrFE)) are promising materials for nonvolatile memory applications. The device is built in the form of themetal–ferroelectric–insulator–semiconductor (MFIS) capacitance memory. The results demonstrated that LB films can provide devices with low-voltage operation.[10]
Transducers[edit]
The ferroelectric effect always relates the various force to electric properties, which can be applied in transducers. The flexibility and low cost of polymers facilitates the application of ferroelectic polymers in transducers. The device configuration is simple, it usually consists of a piece of ferroelectric film with an electrode on the top and bottom surfaces. Contacts to the two electrodes complete the design.[11]
Sensors[edit]
When the device functions as a sensor, a mechanical or acoustic force applied to one of the surfaces causes a compression of the material. Via the direct ferroelectric effect, a voltage is generated between the electrodes.
Actuators[edit]
In actuators, a voltage applied between the electrodes causes a strain on the film through the inverse ferroelectric effect.
Soft transducers in the form of ferroelectric polymer foams have been proved to of great potential.[12]
See also[edit]
References[edit]
- ^ "Ferroelectric Properties of Vinylidene Fluoride Copolymers," by T. Furukawa, in Phase Transitions, Vol. 18, pp. 143-211 (1989).
- ^ a b c d Nalwa, H. (1995). Ferroelectric Polymers (First ed.). New York: Marcel Dekker, INC. ISBN 0-8247-9468-0.
- ^ a b c d e Lovinger, A.J. (1983). Science. 220: 1115–1121.
{{cite journal}}: Missing or empty|title=(help) - ^ a b c Ducharme,S. (2008). 13th International Symposium on Electrets: B0501. doi:10.1109/ISE.2008.4814005.
{{cite journal}}: Missing or empty|title=(help) - ^ a b Atkins, P. (2006). "23". Inorganic Chemistry. Overton, Rourke, Weller, Armstrong (Fourth Edition ed.). New York: W.H. Freeman and Company. pp. 609–610. ISBN 0-7167-4878-9.
{{cite book}}:|edition=has extra text (help) - ^ Tashiro K. (1984). Ferroelectrics. 57: 297.
{{cite journal}}: Missing or empty|title=(help) - ^ Nalwa, H.S. (1991). J. Macromol. Sci. Rev. Macromol. Chem. Phys. 29: 341.
{{cite journal}}: Missing or empty|title=(help) - ^ Kepler, R.G. (1992). Adv. Phys. 41: 1.
{{cite journal}}: Missing or empty|title=(help) - ^ Cais, R.E.; Kometani, J.M. (1985). Macromolecules. 18: 1354–1357.
{{cite journal}}: Missing or empty|title=(help) - ^ Ducharme, D.; Reece, T.J.; Othon, C.M; Rannow, R.K. (2005). IEEE TRANSACTIONS ON DEVICE AND MATERIALS RELIABILITY. 5: 720–733.
{{cite journal}}: Missing or empty|title=(help) - ^ Kressmann, R. (2001). J. Acoust. Soc. Am. 109: 1412.
{{cite journal}}: Missing or empty|title=(help) - ^ Bauer, S.; Gerhard-Multhaupt, R.; Sessler, G.M (2004). Phys. Today. 57: 37–43.
{{cite journal}}: Missing or empty|title=(help)
