User:Airman72
Mixtures[edit]
In 1890 van der Waals published an article that opened the study of fluid mixtures. It was subsequently included as Part III of a later published version of his thesis.[1] His essential idea was that in a binary mixture of vdw fluids described by the equations
For molecules that are hard spheres, , the arithmetic mean of and . Substituting this into the quadratic form, and noting that then produces
Van der Waals also in this article made use of the Helmholtz Potential Minimum Principle, one of a number of alternate extremum princlple of thermodynamics. This one states that in a system in diathermal contact with a heat reservoir , and , namely at equilibrium the Helmholtz potential is a minimimum.[5] This leads to a requirement , which is the same as before. For stable states of a single fluid the curvature of the molar Helmholtz potential, , is positive.
For a single substance the definition of the molar Gibbs free energy can be written in the form . Thus when and are constant the function represents a straight line with slope , and intercept . For a given subcritical, , with and a suitable value of this line is tangent to at the molar volume of each coexisting phase, saturated liquid, , and saturated vapor, . Furthermore, each of these points is characterized by the same value of as well as the same values of and . These are the same three specifications for coexistence that were used previously.

As depicted in Fig. 8, the region on the green curve for ( is designated by the left green circle) is the liquid. As increases past the curvature of (proportional to ) continually decreases. The point characterized by , is a spinodal point, and between these two points is the metastable superheated liquid. For further increases in the curvature decreases to a minimum then increases to another spinodal point; between these two spinodal points is the unstable region in which the fluid cannot exist in a homogeneous equilibrium state. With a further increase in the curvature increases to a maximum at , where the slope is ; the region between this point and the second spinodal point is the metastable subcooled vapor. Finally, the region is the vapor. In this region the curvature continually decreases until it is zero at infinitely large . Neither the spinodal points nor the metastable and unstable regions are distinguished in Fig. 8 as they are in Fig.1.
In the case of a vdW fluid the molar Helmholtz potential can be cast in the form
For a binary mixture of vdW fluids the Helmholtz potential is a function of 2 variables, , where is a composition variable, for example so . In this case there are three stability conditions
For a binary mixture the Euler equation,[7] can be written in the form
a spectacular vindication of the essential physical correctness of the ideas behind the van der Waals equation, for almost every kind of critical behavior found in practice can be reproduced by the calculations, and the range of parameters that correlate with the different kinds of behavior are intelligible in terms of the expected effects of size and energy.[11]
In order to obtain these numerical results, in addition to the values of the constants of the individual component fluids that must be known, the effect of collisions between molecules of the different components, given by and , must be specified. In he absence of experimental data, or computer modelling results to estimate their value, the empirical combining laws
If a mixture of gases is being considered, and each gas has its own (attraction between molecules) and (volume occupied by molecules) values, then and for the mixture can be calculated as [4]
- = total number of moles of gas present,
- for each , = number of moles of gas present, and
- [14] or [15]
- [14] or [15]
and the rule of adding partial pressures becomes invalid if the numerical result of the equation is significantly different from the ideal gas equation .
Mathematical details[edit]
In terms of the right ascension of the Sun, α, and that of a mean Sun moving uniformly along the celestial equator, αM, the equation of time is defined as the difference,[16] Δt = αM - α. In this expression Δt is the time difference between apparent solar time (time measured by a sundial) and mean solar time (time measured by a mechanical clock). The left side of this equation is a time difference while the right side terms are angles; however, astronomers regard time and angle as quantities that are related by conversion factors such as; 2π radian = 360° = 1 day = 24 hour. The difference, Δt, is measureable because α can be measured and αM, by definition, is a linear function of mean solar time.
The equation of time can be calculated based on Newton's theory of celestial motion in which the earth and sun describe elliptical orbits about their common mass center. In doing this it is usual to write αM = 2πt/tY = Λ where
- t is dynamical time, the independent variable in the theory,
- tY is the length of time in a tropical year,
- Λ is the ecliptic longitude of a dynamical mean Sun; the angle from the vernal equinox to a fictitious Sun moving uniformly along the ecliptic and coinciding with the apparent sun at apoapsis and periapsis.
Substituting αM into the equation of time, it becomes[17]
The new angles appearing here are:
- M is the mean anomaly; the angle from the periapsis to the dynamical mean Sun,
- λp = Λ - M = 4.9412 = 283.11° is the ecliptic longitude of the periapsis written with its value on 1 Jan 2010 at 12 noon.
However, the displayed equation is approximate; it is not accurate over very long times because it ignores the distinction between dynamical time and mean solar time[18]. In addition, an elliptical orbit formulation ignores small perturbations due to the moon and other planets. Another complication is that the orbital parameter values change significantly over long times, for example λp increases by about 1.7 degrees per century. Consequently, calculating Δt using the displayed equation with constant orbital parameters produces accurate results only for sufficiently short times (decades). It is possible to write an expression for the equation of time that is valid for centuries, but it is necessarily much more complex[19].
In order to calculate α, and hence Δt, as a function of M, three additional angles are required; they are
- E the Sun's eccentric anomaly,
- ν the Sun's true anomaly,
- λ = ν + λp the Sun's true longitude on the ecliptic.
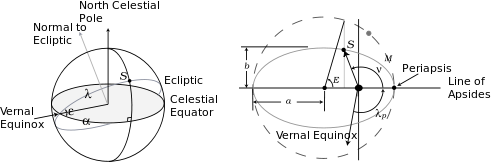
All these angles are shown in the figure on the right, which shows the celestial sphere and the Sun's elliptical orbit seen from the Earth (the same as the Earth's orbit seen from the Sun). In this figure ε = 0.40907 = 23.438° is the obliquity, while e = [1 − (b/a)2]1/2 = 0.016705 is the eccentricity of the ellipse.
Now given a value of 0≤M≤2π, one can calculate α(M) by means of the following procedure:[20]
First, knowing M, calculate E from Kepler's equation[21]
A numerical value can be obtained from an infinite series, graphical, or numerical methods. Alternatively, note that for e = 0, E = M, and for small e, by iteration[22], E ~ M + e sin M. This can be improved by iterating again, but for the small value of e that characterises the orbit this approximation is sufficient.
Next, knowing E, calculate the true anomaly ν from an elliptical orbit relation[23]
The correct branch of the multiple valued function tan−1x to use is the one that makes ν a continuous function of E(M) starting from ν(E=0) = 0. Thus for 0≤ E < π use tan−1x = Tan−1x, and for π < E ≤ 2π use tan−1x = Tan−1x + π. At the specific value E = π for which the argument of tan is infinite, use ν = E. Here Tan−1x is the principal branch, |Tan−1x| < π/2; the function that is returned by calculators and computer applications. Alternatively, note that for e = 0, ν = E and for small e, from a one term Taylor expansion, ν ~ E+e sin E ~ M +2 e sin M.
Next knowing ν calculate λ from its definition above
The value of λ varies non-linearly with M because the orbit is elliptical, from the approximation for ν, λ ~ M + λp + 2 e sin M.
Next, knowing λ calculate α from a relation for the right triangle on the celestial sphere shown above[24]
Like ν previously, here the correct branch of tan−1x to use makes α a continuous function of λ(M) starting from α(λ=0)=0. Thus for (2k-1)π/2 < λ < (2k+1)π/2, use tan−1x = Tan−1x + kπ, while for the values λ = (2k+1)π/2 at which the argument of tan is infinite use α = λ. Since λp ≤ λ ≤ λp+ 2π when M varies from 0 to 2π, the values of k that are needed, with λp = 4.9412, are 2, 3, and 4. Although an approximate value for α can be obtained from a one term Taylor expansion like that for ν[25], it is more efficatious to use the equation[26] sin(α - λ) = - tan2(ε/2) sin(α + λ). Note that for ε = 0, α = λ and for small ε, by iteration, α ~ λ - tan2(ε/2) sin 2λ ~ M + λp + 2e sin M - tan2(ε/2) sin(2M+2λp).
Finally, Δt can be calculated using the starting value of M and the calculated α(M). The result is usually given as either a set of tabular values, or a graph of Δt as a function of the number of days past periapsis, n, where 0≤n≤ 365.242 (365.242 is the number of days in a tropical year); so that
Using the approximation for α(M), Δt can be written as a simple explicit expression, which is designated Δta because it is only an approximation.

This equation was first derived by Milne[27], who wrote it in terms of Λ = M + λp. The numerical values written here result from using the orbital parameter values for e, ε, and λp given previously in this section. When evaluating the numerical expression for Δta as given above, a calculator must be in radian mode to obtain correct values. Note also that the date and time of periapsis (perihelion of the Earth orbit) varies from year to year; a table giving the connection can be found in perihelion.
A comparative plot of the two calculations is shown in the figure on the right. The approximate calculation is seen to be close to the exact one, the absolute error, Err = |(Δt− Δta)|, is less than 45 seconds throughout the year; its largest value is 44.8 sec and occurs on day 273. More accurate approximations can be obtained by retaining higher order terms [28], but they are necessarily more time consuming to evaluate. At some point it is simpler to just evaluate Δt, but Δta as written above is easy to evaluate, even with a calculator, and has a nice physical explanation as the sum of two terms, one due to obliquity and the other to eccentricity. This is not true either for Δt considered as a function of M or for higher order approximations of Δta.
Footnotes[edit]
- ^ van der Waals, pp. 243-282
- ^ Lorentz, H. A., Ann, Physik, 12, 134, (1881)
- ^ Rowlinson ED, p. 68
- ^ a b Redlich, Otto; Kwong, J. N. S. (1949-02-01). "On the Thermodynamics of Solutions. V. An Equation of State. Fugacities of Gaseous Solutions" (PDF). Chemical Reviews. 44 (1): 233–244. doi:10.1021/cr60137a013. Retrieved 2024-04-02. Cite error: The named reference "rw" was defined multiple times with different content (see the help page).
- ^ Callen, p. 105
- ^ Kreysig, E., Differential Geometry, University of Toronto Press, Toronto, pp. 124-128, (1959)
- ^ Callen, pp. 47-48
- ^ van der Waals, pp. 253-258
- ^ Rowlinson ED, pp. 23-27
- ^ van der Waals, Rowlinson ED, pp. 23-27, 64-66
- ^ van der Waals, Rowlinson ED, p. 66
- ^ Hirschfelder, J. O., Curtis, C. F., and Bird, R. B., Molecular Theory of Gases and Liquids, John Wiley and Sons, New York, pp. 252-253, (1964)
- ^ Hirschfelder, J. O., Curtis, C. F., and Bird, R. B., pp. 168-169
- ^ a b Niemeyer, Kyle. "Mixture properties". Computational Thermodynamics. Archived from the original on 2024-04-02. Retrieved 2024-04-02.
- ^ a b Hewitt, Nigel. "Who was Van der Waals anyway and what has he to do with my Nitrox fill?". Maths for Divers. Archived from the original on 11 March 2020. Retrieved 1 February 2019.
- ^ Heilbron p 275, Roy p 45
- ^ Duffett-Smith p 98, Meeus p 341
- ^ Hughes p 1530
- ^ Hughes p 1535
- ^ Duffet-Smith p 86
- ^ Moulton p 159
- ^ Hinch p 2
- ^ Moulton p 165
- ^ Burington p 22
- ^ Whitman p 32
- ^ Milne p 374
- ^ Milne p 375
- ^ Muller Eqs (45) and (46)
References[edit]
- Burington R S 1949 Handbook of Mathematical Tables and Formulas (Sandusky, Ohio: Handbook Publishers)
- Duffett-Smith P 1988 Practical Astronomy with your Calculator Third Edition (Cambridge: Cambridge University Press)
- Heilbron J L 1999 The Sun in the Church, (Cambridge Mass: Harvard University Press|isbn=0-674-85433-0)
- Hinch E J 1991 Perturbation Methods, (Cambridge: Cambridge University Press)
- Hughes D W, et al. 1989, The Equation of Time, Monthly Notices of the Royal Astronomical Society 238 pp 1529-1535
- Meeus, J 1997 Mathematical Astronomy Morsels, (Richmond, Virginia: Willman-Bell)
- Milne R M 1921, Note on the Equation of Time, The Mathematical Gazette 10 (The Mathematical Association) pp 372–375
- Moulton F R 1970 An Introduction to Celestial Mechanics, Second Revised Edition, (New York: Dover)
- Muller M 1995, "Equation of Time - Problem in Astronomy", Acta Phys Pol A 88 Supplement, S-49.
- Roy A E 1978 Orbital Motion, (Adam Hilger|ISBN=0-85274-228-2)
- Whitman A M 2007, "A Simple Expression for the Equation of Time", Journal Of the North American Sundial Society 14 pp 29–33



















































![{\displaystyle f_{r}=u_{r}-T_{r}s_{r}={\mbox{C}}_{u}+T_{r}\{c-{\mbox{C}}_{s}-\ln[T_{r}^{c}(3v_{r}-1)]\}-9/(8v_{r})}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7ca9c416fe60666e9a6910029ae63f3a1feb8cb3)


































![{\displaystyle \nu =2\tan ^{-1}\left[{\sqrt {\frac {1+e}{1-e}}}\tan {\frac {E}{2}}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b487c670d7363dfd4a76b6d98e62aacf31e03a9c)

![{\displaystyle \alpha =\tan ^{-1}[\cos \varepsilon \,\tan \lambda ]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fa499ddffe009ee162d6a4cffad8593fe490b1b4)

![{\displaystyle \Delta t_{a}=-2e\sin M+\tan ^{2}{\frac {\varepsilon }{2}}\,\sin(2M+2\lambda _{p})=[-7.657\sin M+9.862\sin(2M+3.599)]{\mbox{min}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fc3f87a066e613741c0211ba3e210aafbe13b539)