Plum pudding model

The plum pudding model is an obsolete scientific model of the atom. It was first proposed by J. J. Thomson in 1904[1] following his discovery of the electron in 1897, but before Ernest Rutherford discovered the atomic nucleus in 1911. The model tried to account for two properties of atoms then known: that there are electrons and that atoms have no net electric charge. Logically there had to be a commensurate quantity of positive charge to balance out the negative charge of the electrons, but having no clue as to the source of this positive charge, Thomson tentatively proposed it was everywhere in the atom, the atom being in the shape of a sphere. This was the mathematically simplest model to fit the known facts.[2] Following from this, Thomson imagined that the balance of electrostatic forces in the atom would distribute the electrons in a more or less even manner throughout this hypothetical sphere.
Thomson's model is popularly referred to as the plum pudding model with the idea that the electrons are distributed with similar density as raisins in a plum pudding. Neither Thomson nor his colleagues ever used this analogy.[3] It seems to have been a conceit of popular science writers to make the model accessible to the layman. The analogy is perhaps misleading because Thomson likened the sphere to a liquid rather than a solid since he thought the electrons moved around in it.[4]
Overview[edit]
It had been known for many years that atoms contain negatively charged subatomic particles. Thomson called them "corpuscles" (particles), but they were more commonly called "electrons", the name G. J. Stoney had coined for the "fundamental unit quantity of electricity" in 1891.[5] It had also been known for many years that atoms have no net electric charge. Thomson held that atoms must also contain some positive charge that cancels out the negative charge of their electrons.[6][7] Thomson published his proposed model in the March 1904 edition of the Philosophical Magazine, the leading British science journal of the day. In Thomson's view:
... the atoms of the elements consist of a number of negatively electrified corpuscles enclosed in a sphere of uniform positive electrification, ...[8]
Thomson's model was the first to assign a specific inner structure to an atom, though his original description did not include mathematical formulas.[3][9] He had followed the work of William Thomson (later Lord Kelvin) who had written a paper proposing a vortex atom in 1867,[10] J.J. Thomson abandoned his 1890 "nebular atom" hypothesis, based on the vortex theory of the atom, in which atoms were composed of immaterial vortices and suggested there were similarities between the arrangement of vortices and periodic regularity found among the chemical elements.[11] Thomson based his atomic model on known experimental evidence of the day, and in fact, followed Lord Kelvin's lead again as Kelvin had proposed a positive sphere atom a year earlier.[12][13] Thomson's proposal, based on Kelvin's model of a positive volume charge, served to guide future experiments.
The main objective of Thomson's model after its initial publication was to account for the electrically neutral and chemically varied state of the atom.[8] Electron orbits were stable under classical mechanics. When an electron moves away from the center of the positively charged sphere it is subjected to a greater net positive inward force due to the presence of more positive charge inside its orbit (see Gauss's law). Electrons were free to rotate in rings that were further stabilized by interactions among the electrons, and spectroscopic measurements were meant to account for energy differences associated with different electron rings. As for the properties of matter, Thomson believed they arose from electrical effects. He further emphasized the need of a theory to help picture the physical and chemical aspects of an atom using the theory of corpuscles and positive charge.[14] Thomson attempted unsuccessfully to reshape his model to account for some of the major spectral lines experimentally known for several elements.[15] After the scientific discovery of radioactivity, Thomson decided to address it in his model by stating:
... we must face the problem of the constitution of the atom, and see if we can imagine a model which has in it the potentiality of explaining the remarkable properties shown by radio-active substances ...[16]
Thomson's model changed over the course of its initial publication, finally becoming a model with much more mobility containing electrons revolving in the dense field of positive charge rather than a static structure. Despite this, the colloquial nickname "plum pudding" was soon attributed to Thomson's model as the distribution of electrons within its positively charged region of space reminded many scientists of raisins, then called "plums", in the common English dessert, plum pudding.[3]
Refutation[edit]
Between 1908 and 1913, Ernest Rutherford, Hans Geiger, and Ernest Marsden collaborated on a series of experiments in which they bombarded metal foils with a beam of alpha particles so as to study how the foils scattered said beam. Gold was their preferred material because gold is very malleable and can therefore be made into an especially thin foil. The found that the gold foil could scatter alpha particles by more than 90 degrees. This should not have been possible according to the Thomson model. The scattering should have been negligible. The positive charge in the Thomson model is too diffuse to produce an electric field of sufficient strength to cause such scattering. Rutherford deduced that the positive charge of the atom, along with most of the atom's mass, was concentrated in a tiny nucleus at the center of the atom. Only such an intense concentration of charge, anchored by its high mass, could have scattered the alpha particle beam so dramatically.
Related scientific problems[edit]
As an important example of a scientific model, the plum pudding model has motivated and guided several related scientific problems.
Mathematical Thomson problem[edit]
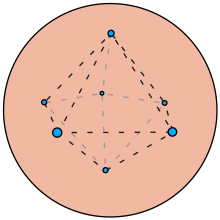
A particularly useful mathematics problem related to the plum pudding model is the optimal distribution of equal point charges on a unit sphere, called the Thomson problem. The Thomson problem is a natural consequence of the plum pudding model in the absence of its uniform positive background charge.[17][18]
Origin of the nickname[edit]

The first known writer to compare Thomson's model to a plum pudding was an anonymous reporter who wrote an article for the British pharmaceutical magazine The Chemist and Druggist in August 1906.
While the negative electricity is concentrated on the extremely small corpuscle, the positive electricity is distributed throughout a considerable volume. An atom would thus consist of minute specks, the negative corpuscles, swimming about in a sphere of positive electrification, like raisins in a parsimonious plum-pudding, units of negative electricity being attracted toward the centre, while at the same time repelling each other.[19]
The analogy was never used by Thomson nor his colleagues. It seems to have been a conceit of popular science writers to make the model easier to understand for the layman.[3]
References[edit]
- ^ "Plum Pudding Model". Universe Today. 27 August 2009. Retrieved 19 December 2015.
- ^ J. J. Thomson (1907). The Corpuscular Theory of Matter, p. 103: "In default of exact knowledge of the nature of the way in which positive electricity occurs in the atom, we shall consider a case in which the positive electricity is distributed in the way most amenable to mathematical calculation, i.e., when it occurs as a sphere of uniform density, throughout which the corpuscles are distributed."
- ^ a b c d Giora Hon; Bernard R. Goldstein (6 September 2013). "J. J. Thomson's plum-pudding atomic model: The making of a scientific myth". Annalen der Physik. 525 (8–9): A129–A133. doi:10.1002/andp.201300732.
- ^ J. J. Thomson, in a letter to Oliver Lodge dated 11 April 1904, quoted in Davis & Falconer (1997):
"With regard to positive electrification I have been in the habit of using the crude analogy of a liquid with a certain amount of cohesion, enough to keep it from flying to bits under its own repulsion. I have however always tried to keep the physical conception of the positive electricity in the background because I have always had hopes (not yet realised) of being able to do without positive electrification as a separate entity and to replace it by some property of the corpuscles."
- ^ O'Hara, J. G. (March 1975). "George Johnstone Stoney, F.R.S., and the Concept of the Electron". Notes and Records of the Royal Society of London. 29 (2): 265–276. doi:10.1098/rsnr.1975.0018. JSTOR 531468. S2CID 145353314.
- ^ "Discovery of the electron and nucleus (article)". Khan Academy. Retrieved 9 February 2021.
- ^ Alviar-Agnew, Marissa; Agnew, Henry (4 April 2016). "4.3: The Nuclear Atom". Introductory Chemistry. LibreTexts. Retrieved 9 February 2021.
- ^ a b Thomson, J. J. (March 1904). "On the Structure of the Atom: an Investigation of the Stability and Periods of Oscillation of a number of Corpuscles arranged at equal intervals around the Circumference of a Circle; with Application of the Results to the Theory of Atomic Structure". Philosophical Magazine. Sixth series. 7 (39): 237–265. doi:10.1080/14786440409463107. Archived (PDF) from the original on 2022-10-09.
- ^ Thomson, J. J. (December 1899). "On the masses of the ions in gases at low pressures". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 48 (295): 547–567. doi:10.1080/14786449908621447.
- ^ Thomson, William (1869). "On Vortex Atoms". Proceedings of the Royal Society of Edinburgh. 6: 94–105. doi:10.1017/S0370164600045430.
- ^ Kragh, Helge (2002). Quantum Generations: A History of Physics in the Twentieth Century (Reprint ed.). Princeton University Press. pp. 43–45. ISBN 978-0691095523.
- ^ Models of the Atom, Michael Fowler, University of Virginia https://galileo.phys.virginia.edu/classes/252/more_atoms.html#Plum%20Pudding
- ^ Kumar, Manjit, Quantum Einstein, Bohr and the Great Debate, ISBN 978-0393339888, 2008.
- ^ E. R. (1908). "The Corpuscular Theory of Matter". Nature. 77 (2005): 505–506. Bibcode:1908Natur..77..505R. doi:10.1038/077505a0. S2CID 36356538.
- ^ Models of the Atom, Michael Fowler, University of Virginia https://galileo.phys.virginia.edu/classes/252/more_atoms.html#Plum%20Pudding
- ^ Thomson, J. J. (1904). Electricity and Matter. Mrs. Hepsa Ely Silliman Memorial Lectures. New Haven: Yale University Press. ISBN 978-0-686-83533-2.
- ^ Levin, Y.; Arenzon, J. J. (2003). "Why charges go to the Surface: A generalized Thomson Problem". Europhys. Lett. 63 (3): 415–418. arXiv:cond-mat/0302524. Bibcode:2003EL.....63..415L. doi:10.1209/epl/i2003-00546-1. S2CID 250764497.
- ^ Roth, J. (2007-10-24). "Description of a highly symmetric polytope observed in Thomson's problem of charges on a hypersphere". Physical Review E. 76 (4): 047702. Bibcode:2007PhRvE..76d7702R. doi:10.1103/PhysRevE.76.047702. ISSN 1539-3755. PMID 17995142.
Although Thomson's model has been outdated for a long time by quantum mechanics, his problem of placing charges on a sphere is still noteworthy.
- ^ "What is Matter?". The Chemist and Druggist. 69 (8): 329–330. 25 August 1906.
Bibliography[edit]
- J. J. Thomson (1907). The Corpuscular Theory of Matter. Charles Scribner's Sons.
