Diethofencarb
| |
| Names | |
|---|---|
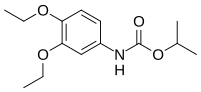
| Preferred IUPAC name
propan-2-yl N-(3,4-diethoxyphenyl)carbamate | |
| Other names
Diethofencarb
| |
| Identifiers | |
3D model (JSmol)
|
|
| 8393454 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.118.674 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H21NO4 | |
| Molar mass | 267.325 g·mol−1 |
| Hazards | |
| GHS labelling:[1] | |

| |
| Warning | |
| H319 | |
| P264+P265, P280, P305+P351+P338, P337+P317 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diethofencarb is a carbamate fungicide which is used to control Botrytis infections on a variety of fruit and vegetable crops.[2][3]
References[edit]
- ^ "Diethofencarb". pubchem.ncbi.nlm.nih.gov.
- ^ Leroux P, Fritz R, Debieu D, Albertini C, Lanen C, Bach J, et al. (September 2002). "Mechanisms of resistance to fungicides in field strains of Botrytis cinerea". Pest Management Science. 58 (9): 876–888. doi:10.1002/ps.566. PMID 12233177.
- ^ Liu YH, Yuan SK, Hu XR, Zhang CQ (August 2019). "Shift of Sensitivity in Botrytis cinerea to Benzimidazole Fungicides in Strawberry Greenhouse Ascribing to the Rising-lowering of E198A Subpopulation and its Visual, On-site Monitoring by Loop-mediated Isothermal Amplification". Scientific Reports. 9 (1): 11644. Bibcode:2019NatSR...911644L. doi:10.1038/s41598-019-48264-4. PMC 6690993. PMID 31406191.
